Instructor Preparation
advertisement

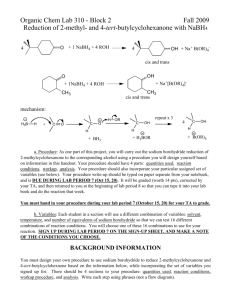
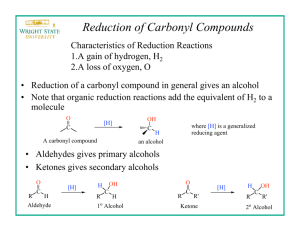
Equipment & Chemical Preparation Sodium Borohydride Reduction of 2-Methylcyclohexanone In this experiment, students will work in pairs to: 1. synthesize 2-methylcyclohexanol from 2-methylcyclohexanone using NaBH4 2. weigh the product & determine a % yield 3. analyze the product by IR spectroscopy 4. analyze the product using a gas chromatogram provided to them Each student will synthesize 2-methylcyclohexanol starting with 1.2g of 2methylcyclohexanone, 200mg of NaBH4 and 10 ml of methanol. There are ~150 students. The reaction is carried out in 50 ml Erlenmeyer flasks, immersed in an ice bath with stirring. The work-up of the reaction involves addition of 5 ml of 3M NaOH and 5 ml of deionized water, followed by extraction in a separatory funnel with CH2Cl2 (~35 ml each). The CH2Cl2 layer that is separated in the extraction step is dried with ~100mg of MgSO4. The product is analyzed by IR spectroscopy and gas chromatography (provided). The total materials need for the experiment are listed in the table below. Chemicals 2-Methylcyclohexanone Sodium borohydride (NaBH4) Methanol 3M NaOH Deionized water Magnesium sulfate CH2Cl2 Equipment 50 ml Erlenmeyer flasks Crystallizing dishes (for ice/water baths) (Note Size: 125mm X 65 mm) Magnetic stir bars Salt Plates (for IR spectroscopy) Disposable Pasteur pipettes ~300g ~50g ~4 liters ~3 liters ~3 liters ~50g ~4 liters 24 12 24 6 sets 2-3 boxes Six bins should be prepared for each of the six lab benches containing the following items listed in the table below. Quantities should be replenished throughout the week. Experiment Bin (1 per bench) 2-methylcyclohexanone NaBH4 Methanol Deionized Water 3M NaOH Dichloromethane MgSO4 Magnetic Stir bars 50ml Erlenmeyer flasks Crystallizing dishes Salt plates ~30g (30ml) ~5g ~300ml ~200ml ~200ml ~1 liter ~4g 4 4 2 1 set