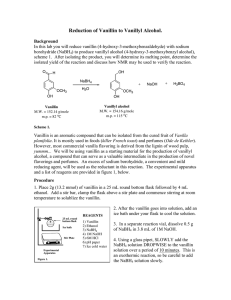
Borohydride Reduction of Vanillin to Vanillyl Alcohol

Borohydride Reduction of Vanillin to Vanillyl Alcohol
Introduction:
The purpose of this experiment is to develop a method to carry out the synthesis of vanillyl alcohol from vanillin. Organic chemists attempt to synthesize compounds regularly and do not have a set procedure to follow. When synthesizing a new compound they have several options; adapt and modify existing procedures, come up with a new procedure base on what knowledge they have of the product and starting materials, and of course a chemist can invent a new procedure for synthesizing a desired compound.
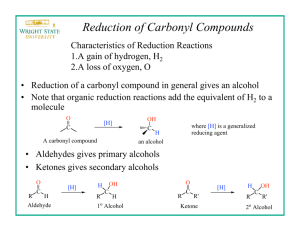
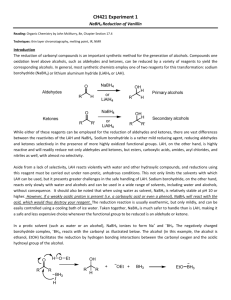
One of the key words for this reaction is reduction. Reduction implies that a compound will lose oxygen atoms and or gain hydrogen atoms. This can be accomplished with a reducing agent. Historically lithium aluminum hydride was introduced as a reducing agent back in the
1940s. This agent brought about a revolution in the synthesis of alcohols via reduction. Hydride reduction eventually became the norm due to simplicity and convenience. Lithium aluminum hydride is a powerful reducing agent however it reacts violently with water and other hydroxylic solvents. It can only be used in solvents such as diethyl ether (aprotic solvents) under strictly anhydrous conditions. It is also expensive and hazardous to use. The solution to this is another reducing agent, sodium borohydride. Sodium borohydride is a much milder reducing agent and is safer to handle. NaBH4 can even be used in aqueous or alcohol solution which is an advantage over LiAlH4.
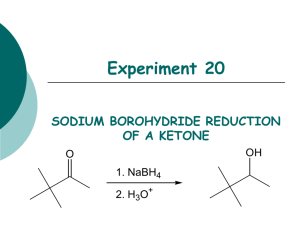
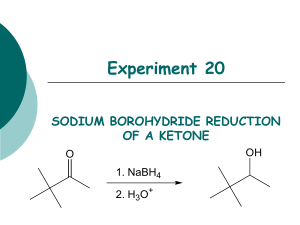
The reaction carried out is a reduction of an aldehyde function of vanillin to a primary alcohol using sodium borohydride in a basic aqueous medium.
Results:
Mechanism:
Sodium Borohydride Reduction
Physical Properties
Calculations:
The amounts of each reactant were given in the lab.
% difference in mp = (115°C – 87°C)/ 100 = 28%
Discussion:
As mentioned in the introduction. The purpose of this experiment was to find an optimal method for carrying out the synthesis of Vanillyl Alcohol via a reduction using NaBH4 as a reducing agent. After completion of the reaction, several tests are done to determine how close the product is to the literature results.
First a ring stand, clamp, round bottom flask, heating plate and separatory addition funnel are gathered. While this is being setup, 3.8 g of vanillin is measured out and added to the reaction flask. The amount of solvent required should not be in excess but just enough to dissolve the reactants. A suitable solvent for this reaction is NaOH for its basic properties that are essential for this experiment (which will be explained in brief). 30 ml of NaOH is gathered for use as a solvent and is added to the reaction flask (round bottom). A magnetic stir is dropped into the flask and the apparatus is placed over a heat source and the stir option is then turned on. Stirring is done to ensure that the solid has dissolved completely.
This reaction is a reduction reaction, a reduction can be considered as the addition of hydrogen
(protonation) and/or the removal of oxygen which would change the oxidation state of a molecule and or atom;
In this case, sodium borohydride (NaBH4) is the reducing agent. Vanillin is reacted with NaBH4 and is reduced to an alcohol. NaBH4 isn’t stable at low pH and decomposes at a rate of 4.5% per hour at 25°C in neutral aqueous solution. NaOH is used to neutralize the carbonyl compound and maintain the pH at 10. 0.4 g of NaBH4 is measured out and dissolved in solvent. It is then put in the separatory addition funnel and added drop-wise to the reaction flask. It is important to keep temperatures down so as to not merit unwanted reactions; therefore as the NaBH4 is added it was kept in cool water. The solution turns yellow once the NaBH4 is added and the reaction will continue for roughly 30 minutes. It is important to note that NaBH4 reacts slowly with alcohols and that most reactions involving of aldehydes and aliphatic ketones require 30 minutes to reach completion at room temperature. However aromatic ketones may require more time because they are rather hindered ketones.
HCL is then added drop wise to precipitate the solution. A test for acidity is then carried out to ensure that not too much HCl is present.
The mixture Is then recrystillized and separated through vacuum filtration. And dried using vacuum filtration
The recorded mp of 87°C in indicates that the product obtained contains impurities of
Vanillin where the melting point is 79°C, since the products mp is closer to that of Vanillin it is possible that the reaction had not been brought to completion. This may have resulted from not letting the reaction go for long enough or not having dissolved the reactant completely. It should also be notted that there is a possibility of the use of too much NaBH4 which may have remained in the product.
From the NMR Spec, there’s a strong signal at 4p.p.m indicating the presence of an alkyl halide group. This may be an indication of the sodium borohydride not having reacted completely with the solute, meaning that a large amount of reactant and solvent were still present in the product.