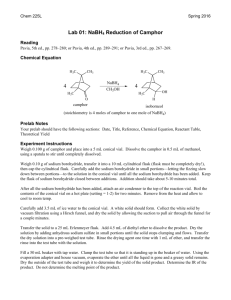
NaBH4 Reduction of Cyclohexanone: Organic Chemistry Lab
advertisement

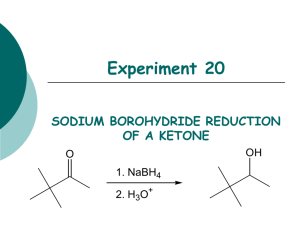
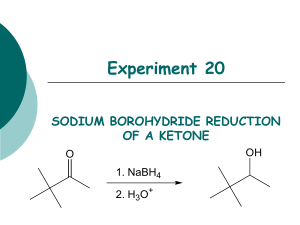
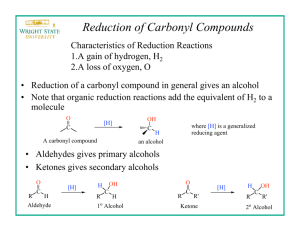
Organic Chem Lab 310 - Block 2 Fall 2009 Reduction of 2-methyl- and 4-tert-butylcyclohexanone with NaBH4 O + 1 NaBH4 + 4 ROH 4 OH + Na+ B(OR)4- 4 cis and trans OH O + Na+[B(OR)4]- + 1NaBH4 + 4 ROH CH3 cis and trans CH3 mechanism: H3B H + C C O O H + BH3 H repeat x 3 OR C OH H + H3BOR C 4 OH H + B(OR)4 a. Procedure: As one part of this project, you will carry out the sodium borohydride reduction of 2-methylcyclohexanone to the corresponding alcohol using a procedure you will design yourself based on information in this handout. Your procedure should have 4 parts: quantities used, reaction conditions, workup, analysis. Your procedure should also incorporate your particular assigned set of variables (see below). Your procedure write-up should be typed on paper separate from your notebook, and is DUE DURING LAB PERIOD 7 (Oct 15, 20). It will be graded (worth 14 pts), corrected by your TA, and then returned to you at the beginning of lab period 8 so that you can tape it into your lab book and do the reaction that week. You must hand in your procedure during your lab period 7 (October 15, 20) for your TA to grade. b. Variables: Each student in a section will use a different combination of variables: solvent, temperature, and number of equivalents of sodium borohydride so that we can test 16 different combinations of reaction conditions. You will choose one of these 16 combinations to use for your reaction. SIGN UP DURING LAB PERIOD 7 ON THE SIGN-UP SHEET, AND MAKE A NOTE OF THE CONDITIONS YOU CHOOSE. BACKGROUND INFORMATION You must design your own procedure to use sodium borohydride to reduce 2-methylcyclohexanone and 4-tert-butylcyclohexnone based on the information below, while incorporating the set of variables you signed up for. There should be 4 sections to your procedure: quantities used, reaction conditions, workup procedure, and analysis. Write each step using phrases (not a flow diagram). 1. QUANTITIES USED (g or mL): Note the stoichiometry - in theory, 1.0 mmol of ketone requires 0.25 mmol of NaBH4. However, excess NaBH4 allows for some decomposition of the hydride and also helps drive the reaction to completion. Everyone should start with 16 mmol of the ketone. In this case, 1 equivalent of NaBH4 would be 4 mmol, so 8 equivalents of NaBH4 are 32 mmol, and 12 equivalents are 48 mmol. The number of equivalents of NaBH4 will be one of the variables, either 4, or 8 or 12 equivalents. 2-methylcyclohexanone [583-60-8] MW=112, bp = 165°, d = 0.924, insoluble in water, soluble in organic solvents; unpleasant odor. Note: to help control the odor of the cyclohexanone, open the bottle only in your own hood and rinse the graduated cylinder after use with several portions of acetone into a waste container (e.g. an Erlenmyer) in the hood. Cis-2-methylcyclohexanol [7443-70-1] MW=114, colorless oil, mp 6-8°, bp 165°, slightly soluble in water, soluble in organic solvents. Trans 2-methylcyclohexanol [7443-52-9] MW=114, colorless oil, mp -21°, bp 167.5°, slightly soluble in water, soluble in organic solvents. 4-tert-butylcyclohexanone [98-53-3] MW=154, mp 47-50°, bp 113-116 @20 mm Hg, insoluble in water, soluble in organic solvents, unpleasant odor – work in hood as above. 4-tert-butylcyclohexanol [98-52-2] MW=156, white solid, mp 62-70°, bp 110-115 @ 15 mm Hg°, insoluble in water, soluble in organic solvents. sodium borohydride [16940-66-2] MW=38, white solid, mp 400°, soluble in water and organic solvents. Hydride reducing agents were first introduced in the late 1940's. As opposed to lithium aluminum hydride (LiAlH4), sodium borohydride (NaBH4) is a mild reducing agent that is relatively safe to handle as a solid, and can even be used in aqueous solutions in the absence of any acid. It is particularly useful for selectively reducing aldehydes and ketones since it is unreactive to many other functional groups. Note: It will be convenient to add the dry NaBH4 slowly to your solution of ketone, since heat will be released. 2. REACTION CONDITIONS: Solvent: NaBH4 reductions are carried out in water or an alcohol like methanol, ethanol, or isopropanol. You should be able to figure out why water is not appropriate for this ketone and why isopropanol is not suitable for our laboratory equipment. Both methanol and ethanol can work, so this will be one of our variables. Below is a table showing various properties of methanol and ethanol. pH: pH should not affect the reduction itself very much, but does affect the sodium borohydride. NaBH4 is not stable at low pH (H- + H+ produces H2 gas), and decomposes slowly in neutral conditions; it is stable in basic conditions. In general, the more acidic the solution, the more quickly the sodium borohydride decomposes. A convenient way to prepare basic solutions is to add 1 drop of sodium methoxide to the methanol or ethanol you are using as solvent. methanol ethanol Molecular weight 32 46 Melting point -97° -117° Boiling point 64.7° 78.3° Density, g/mL 0.792 0.789 Water solubility, g/100 m infinite infinite pKa 15.5 15.9 Refractive index 1.329 1.361 Solubility of NaBH4 16.4g 4.0g Temperature: Reactions generally go faster the higher the temperature. However, most reductions like these work well at room temperature (25°C), although sometimes, aromatic or very hindered ketones require higher temperatures. On the other hand, higher temperatures may decompose the NaBH4, leading to incomplete reactions. This year we will compare the use of either 25° or an ice bath. Time: We found in the past that time made very little difference, so everyone will use 30 minutes. 3. WORKUP – what you do after the reaction is complete (difficult part) The first step is to SLOWLY decompose any unreacted NaBH4, using 3 M HCl until the pH is 6. In theory, if the product is a solid, it could be isolated by filtration, evaporation of the solvent, or extraction. Think carefully about this question and consider the following when designing your procedure: 1) Borate salts can form that are soluble in water, partially soluble in methanol or ethanol, and insoluble in methylene chloride - you do not want such salts to contaminate your final product. 2) Many solids do not crystallize easily. 3) Methanol and ethanol are miscible with both water and methylene chloride, so that water and methylene chloride layers do not separate in the presence of the alcohols. Devise a convenient workup procedure that will result in reasonably pure material in the bottom of a flask. Pay attention to what form your product will take (solid or liquid) if it has been heated on the rotary evaporator! Also, pay attention to what form your product will take if it is actually half starting material! 4. ANALYSIS (difficult part) The purpose of the analysis is to determine (A) if the reaction went to completion and (B) what the cis/trans product ratio is. This will be done with the crude products that you isolate in section 3. Divide your Analysis in three parts: (A) Completion of reaction, (B) Ratio of product isomers and (C) 1H NMR assignment of cis- and trans-isomers. (A) Completion of reaction Consider only the methods you have used in this lab. If you choose proton NMR, you should keep in mind that there may be only a very small amount of educt or none left (almost complete or complete reaction). The 1H-NMR signals of the educt will be strongly overlapping with those of the two product isomers and it may be very difficult to identify the former. There may be better ways to do this. (B) Ratio of product isomers You certainly want to use 1H NMR spectroscopy of the crude products to determine the ratio. In order to do this, you need one multiplet of each isomer, that is well separated from other multiplets of products and possible unreacted educt. All protons of educt and product will be cramped together in the region between ~1 and 2.5 ppm, except for the CH-OH protons of the two product isomers, which will be somewhere in the region between 3-4 ppm. Furthermore, an axial cyclohexane proton will typically have a substantially different chemical shift (∆δ ~ 0.5 ppm) than an equatorial proton. (C) 1H NMR assignment of cis- and trans-isomers You will have to use 1H NMR spectroscopy to assign each of the two multiplets in section (B) to either the cis- or the trans-isomer. Many synthetic organic chemists, who synthesize substituted cyclohexanes or complex natural products with one or multiple saturated ringsystems, face this problem almost on a daily basis. They would use the vicinal (3-bond) coupling constants 3J between the CH-OH proton and the protons at the neighboring carbon in the major conformer of the cyclohexane to determine the stereochemistry, that is whether the proton is axial or equatorial. This is possible, because vicinal coupling constants depend on the dihedral angle as shown in the Karplus-curve below. With this information, you should be able to predict the multiplicity and the vicinal coupling constants 3J of the CH-OH proton of each isomer. HΦ H' Φ H C C H' Figure 1: Karplus curve of the dihedral angle dependence of the vicinal H,H-coupling constant 3J. This dependence was predicted theoretically by Prof. M. Karplus (Harvard) () and is consistent with experimental results (shaded area)