18 reducing a ketone using sodium borohydride
advertisement

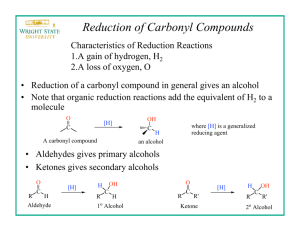
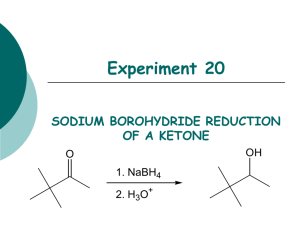
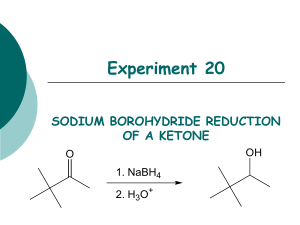
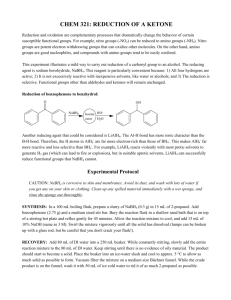
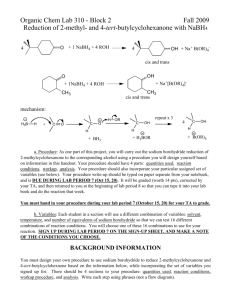
Experiment 5: Reducing a ketone using NaBH4 Risk: Class B This experiment needs close supervision by the teacher because there are significant risks due to the nature of the chemicals or equipment used. Wear goggles and lab coat. Mop up spills using water. Wash hands thoroughly afterwards. Read the hazard warning labels carefully and treat accordingly. Do not carry out the experiment until you have the permission and attention of your teacher. NaBH4 is properly known as sodium tetrahydroborate(III); it is commonly called sodium borohydride. The method involves dissolving the carbonyl compound in warm ethanol (contains about 10 % water). The NaBH4 is added, the mixture stirred and left for 10 to 15 minutes – heat is usually evolved. Water is added and the mixture boiled - this destroys the excess NaBH4. These reactions happen in 2 steps: a) Step 1: initiated by the nucleophilic attack of an H- to the C of the carbonyl group, b) Step 2: followed by the attack of a H+ ion from water to the anion. NaBH4 Overall: R1COR2 + 2 H ( H- + H+ ) R1CH(OH)R2 H2O / heat The mechanism is: 1) Add 0.7g of 1,2-diphenylethanedione (Benzil) to 7cm3 of ethanol in a small conical flask 2) Warm the mixture in a hot water bath at about 50oC until the solid dissolves. 3) Cool. When the solid reappears as fine suspension add 0.15g sodium tetahydroborate (III). Allow to stand for 10 minutes. 4) Add 15cm3 of distilled water and heat until the water boils. Allow the mixture to cool. 5) Collect the crystals by suction (Buchner) filtration. Suck dry and wash with 100cm3 of distilled water. Transfer the solid to a clean filter paper and press dry. 6) Design some chemical experiments to show that the starting material and the product have different functional groups. 7) Write down a balanced equation using displayed formulae and 2H as the reductant.