Temperature_Accuracy_Precision
advertisement

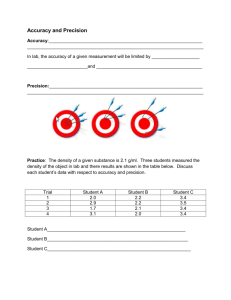
Temperature • Measurement of kinetic energy of atoms or molecules Temperature Scales Water Boils 212 100 373 Water Freezes 32 0 273 - 452 - 273 0 Absolute Zero Fahrenheit (°F) Celsius (°C) – Based on boiling & freezing point of water Kelvin (K) • Kelvin temperature scale – Based on absolute zero – Absolute zero is when all motion of atoms stop! 273 + °C = K • Accuracy – How close a measured value is to the accepted value or target • Precision – How close a series of measurements are to one another 1 2 3 4 Accurate? Precise? Accurate = means close to the bull’s eye Precise = means shots are close to one another Practice • Density of water is 1 g/mL • Student one Student 1 – Accurate? – Precise? • Student two – Accurate? – Precise? Student 2 1.03 0.89 0.98 0.89 1.01 0.87 0.99 0.88 Percent Error • Ratio of error to the accepted value • Can be positive or negative • We like to within 5% error % error 100 accepted value (experimental accepted ) % error 100 accepted value Percent Error Practice Problems 1. The boiling point of water is 100°C. During an experiment, water came to a boil at 97°C according to the thermometer that was being used. What is the percent error? % error error 100 accepted value (experimental accepted ) % error 100 accepted value Percent Error Practice Problems 2. An experiment was performed to determine the density of water. The results of the experiment showed that water had a density of 1.15 g/mL(real value is 1.0 g/mL). What is the percent error? % error error 100 accepted value (experimental accepted ) % error 100 accepted value Percent Error Practice Problems 3. An experiment was conducted to find the mass of one mole of carbon atoms. The results of the experiment showed that a mole of carbon atoms has a mass of 15.78g. The actual value is 16 grams. What is the percent error? error % error accepted value 100 (experimental accepted ) % error 100 accepted value Percent Error Practice Problems 4. An experiment performed to determine the density of lead yields a value of 10.95 g/cm3. The actual value for the density of lead is 11.342 g/cm3. Find the percent error. error % error accepted value 100 (experimental accepted ) % error 100 accepted value Percent Error Practice Problems 5. Find the percent error in a measurement of the boiling point of bromine if the experimental value is 40.6°C and the actual value is 59.35°C. % error error 100 accepted value (experimental accepted ) % error 100 accepted value