Dimensional Analysis and Significant Figures Review
advertisement
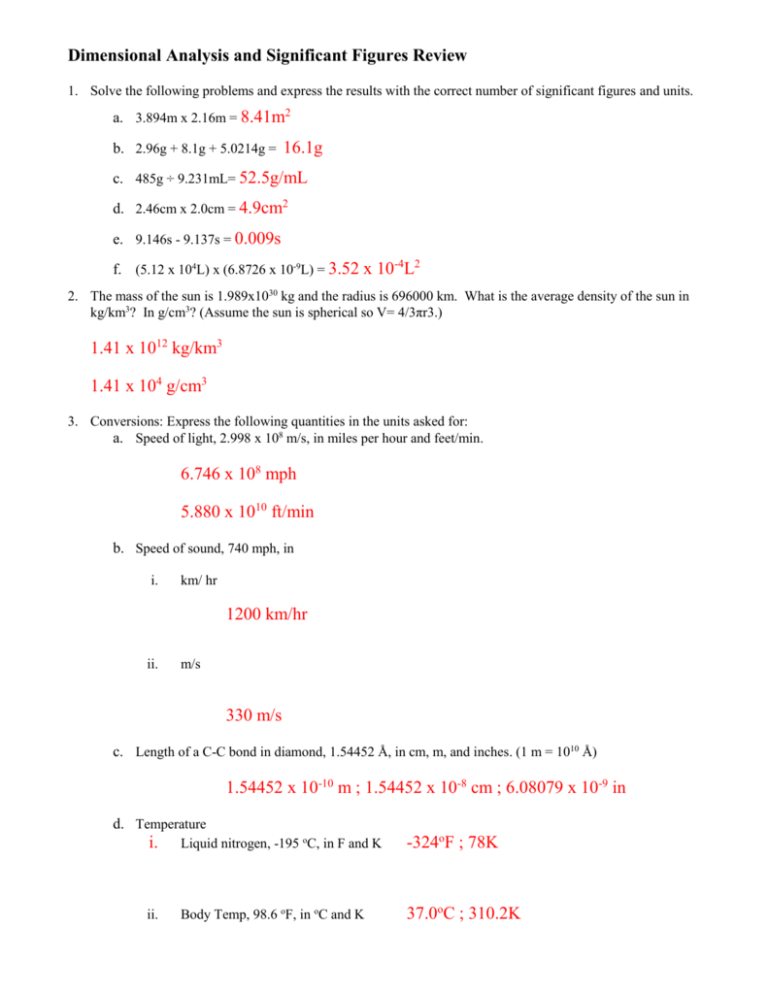
Dimensional Analysis and Significant Figures Review 1. Solve the following problems and express the results with the correct number of significant figures and units. a. 3.894m x 2.16m = 8.41m2 b. 2.96g + 8.1g + 5.0214g = 16.1g c. 485g ÷ 9.231mL= 52.5g/mL d. 2.46cm x 2.0cm = 4.9cm2 e. 9.146s - 9.137s = 0.009s f. (5.12 x 104L) x (6.8726 x 10-9L) = 3.52 x 10-4L2 2. The mass of the sun is 1.989x1030 kg and the radius is 696000 km. What is the average density of the sun in kg/km3? In g/cm3? (Assume the sun is spherical so V= 4/3πr3.) 1.41 x 1012 kg/km3 1.41 x 104 g/cm3 3. Conversions: Express the following quantities in the units asked for: a. Speed of light, 2.998 x 108 m/s, in miles per hour and feet/min. 6.746 x 108 mph 5.880 x 1010 ft/min b. Speed of sound, 740 mph, in i. km/ hr 1200 km/hr ii. m/s 330 m/s c. Length of a C-C bond in diamond, 1.54452 Å, in cm, m, and inches. (1 m = 1010 Å) 1.54452 x 10-10 m ; 1.54452 x 10-8 cm ; 6.08079 x 10-9 in d. Temperature i. Liquid nitrogen, -195 oC, in F and K -324oF ; 78K ii. Body Temp, 98.6 oF, in oC and K 37.0oC ; 310.2K 4. What is the number of atoms present in 2.0 moles of helium gas? 1.2 x 1024 atoms 5. Convert 3.770X1027 atoms of gold to moles of gold. 6.262 x 103 moles 6. How many significant figures are in each of the following? a. 1.92 mm 3 b. 0.030100 kJ 5 c. 6.022 x10 atoms 4 h. 0.001g 5 3 e. 0.00036 cm 3 g. 1001 seconds 23 d. 460.00 L f. 100 people i. 0.01 m 4 1 1 2 7. Record the following in correct scientific notation: a. 350,000,000 cal 3.5 x 108 b. 0.0000721 mol c. 0.0000000809 Ǻ d. 765,400,000,000 atoms 7.21 x 10-5 8.09 x 10-8 7.654 x 1011 Don’t forget to study about matter and the changes it undergoes (including properties and classification), as well as accuracy, precision and percent error.