Ch2
Chapter 2 Worksheet – Dr. Steel
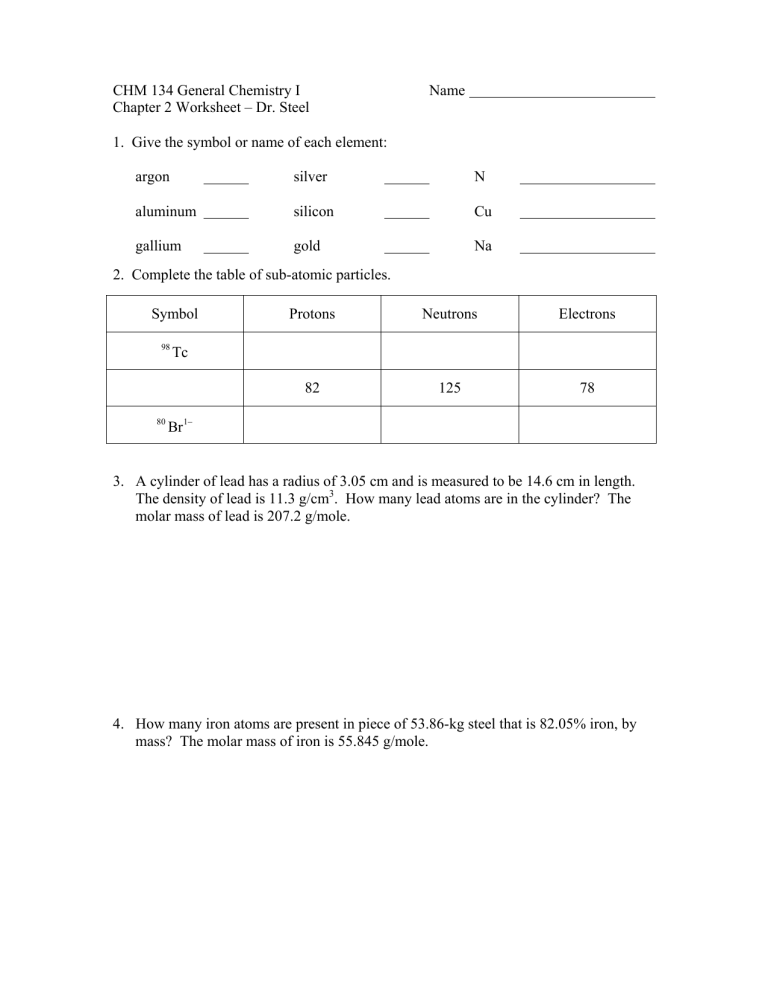
1. Give the symbol or name of each element:
argon silver
aluminum silicon
gallium gold
2. Complete the table of sub-atomic particles.
N
Cu
Na
Symbol Protons Neutrons Electrons
98
Tc
80 1
Br
3. A cylinder of lead has a radius of 3.05 cm and is measured to be 14.6 cm in length.
The density of lead is 11.3 g/cm
3
. How many lead atoms are in the cylinder? The molar mass of lead is 207.2 g/mole.
4. How many iron atoms are present in piece of 53.86-kg steel that is 82.05% iron, by mass? The molar mass of iron is 55.845 g/mole.
5. The relative abundances of magnesium’s three naturally occurring isotopes are shown in the table below. Calculate the atomic mass of magnesium.
Isotope Atomic Mass (amu) Abundance
Mg-24 23.9850 78.99
Mg-25 24.9858 10.00
Mg-26 25.9826 11.01
6. A sample of barium metal contains 1.42×10
23
atoms of barium and occupies 8.96 cm
3 of volume. Determine the density of barium.
Answers: 1) Argon (Ar), Silver (Ag), Aluminum (Al), Silicon (Si), Gallium (ga), Gold (Au) N (nitrogen), Cu (copper),
Na (sodium) 3) 1.40×10
25
atoms 4) 4.764×10
26
atoms 5) 24.31 amu 6) 3.62 g/cm
3
Table for #2:
Symbol Protons Neutrons Electrons
98
Tc
207
Pb
4+
80
Br
1
−
43 55 43
82 125 78
35 45 36