Calculating Percent Error What is it??
advertisement

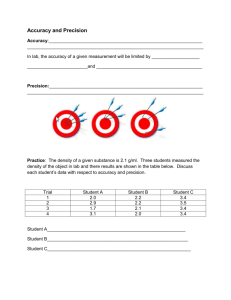
Calculating Percent Error What is it?? Percent Error is used to determine the inaccuracy, in percentage, of a measured or estimated value, compared to an accepted value. The Components of the Formula Estimated Value (Measured Value)- The value that has been derived from an experiment, or an estimated value. Actual Value (What you should have gotten!)- This value is the exact value excepted throughout the scientific community, or the value which is determined exact, at a later point in time. The formula The Formula for Percent Error is a follows: (EV – AV) X 100 = Percent Error AV Remember: EV= Estimated Value (measured value!) AV= Actual Value PS. We multiply by 100 to make to make the decimal a percent. What does it mean if the PE is positive or negative ? Negative if your measurement is too small Positive if your measurement is too big Practice Problems Johnny calculates from an experiment that he has 22.7 grams of carbon. However, he should have gotten 21.8 grams of carbon, which was the accepted value for this experiment. What was his percent error? Remember: (EV-AV) X 100 = PE AV Answer (EV-AV) X 100 = PE AV (22.7-21.8) X 100 = PE 21.8 .900 X 100 = PE 21.8 .0413 X 100 = PE 4.13 % = PE More Practice The actual value for the absorption of carbon is .135. Steven calculated that absorption was .235. What is the percent error in Steven’s experiment? Answer (EV-AV) X 100 = PE AV (.235 - .135) X 100 =PE .135 .100 X 100 = PE .135 .741 X 100 = PE 74.1 % = PE Additional practice 1. The boiling point of water is 100°C. During an experiment, water came to a boil at 97°C according to the thermometer that was being used. What is the percent error of the thermometer? answer 3.0% Additional practice 2. An experiment was conducted to find the mass of one mole of carbon atoms. The results of the experiment showed that a mole of carbon atoms had a mass of 15.78 g. The accepted value of a mole of carbon atoms is 16.00 grams. What is the percent error in this experiment? answer 1.38% Additional practice 3. An experiment performed to determine the density of lead yields a value of 10.95 g/cm3. The accepted value for the density of lead is 11.342 g/cm3. Find the percent error. answer 3.46% Additional practice 4. Find the percent error in a measurement of the boiling point of bromine if the laboratory figure is 40.6°C and the accepted value is 59.35°C. answer 31.59 %