ppt - Fccj.us
advertisement
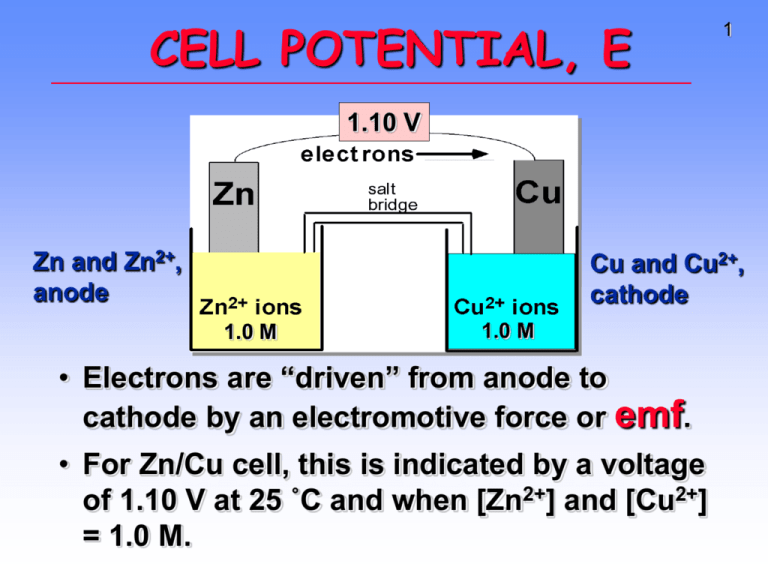
CELL POTENTIAL, E 1 1.10 V Zn and Zn2+, anode Cu and Cu2+, cathode 1.0 M 1.0 M • Electrons are “driven” from anode to cathode by an electromotive force or emf. • For Zn/Cu cell, this is indicated by a voltage of 1.10 V at 25 ˚C and when [Zn2+] and [Cu2+] = 1.0 M. 2 CELL POTENTIAL, E • For Zn/Cu cell, potential is +1.10 V at 25 ˚C and when [Zn2+] and [Cu2+] = 1.0 M. • This is the STANDARD CELL POTENTIAL, Eo • —a quantitative measure of the tendency of reactants to proceed to products when all are in their standard states at 25 ˚C. 3 Calculating Cell Voltage • Balanced half-reactions can be added together to get overall, balanced equation. Zn(s) ---> Zn2+(aq) + 2eCu2+(aq) + 2e- ---> Cu(s) -------------------------------------------Cu2+(aq) + Zn(s) ---> Zn2+(aq) + Cu(s) If we know Eo for each half-reaction, we could get Eo for net reaction. 4 CELL POTENTIALS, Eo Can’t measure 1/2 reaction Eo directly. Therefore, measure it relative to a STANDARD HYDROGEN CELL, SHE. 2 H+(aq, 1 M) + 2e- <----> H2(g, 1 atm) Eo = 0.0 V 5 Zn/Zn2+ half-cell hooked to a SHE. Eo for the cell = +0.76 V Negative electrode Supplier of electrons Zn --> Zn2+ + 2eOxidation Anode Positive electrode Acceptor of electrons 2 H+ + 2e- --> H2 Reduction Cathode Reduction of H+ by Zn 6 Figure 20.10 7 Overall reaction is reduction of H+ by Zn metal. Zn(s) + 2 H+ (aq) --> Zn2+ + H2(g) Eo = +0.76 V Therefore, Eo for Zn ---> Zn2+ (aq) + 2e- is +0.76 V Zn is a (better) (poorer) reducing agent than H2. Cu/Cu2+ and H2/H+ Cell Eo = +0.34 V Positive Acceptor of electrons Cu2+ + 2e- --> Cu Reduction Cathode Negative Supplier of electrons H2 --> 2 H+ + 2eOxidation Anode 8 Cu/Cu2+ and H2/H+ Cell Overall reaction is reduction of Cu2+ by H2 gas. Cu2+ (aq) + H2(g) ---> Cu(s) + 2 H+(aq) Measured Eo = +0.34 V Therefore, Eo for Cu2+ + 2e- ---> Cu is +0.34 V 9 Zn/Cu Electrochemical Cell 10 + Anode, negative, source of electrons Cathode, positive, sink for electrons Zn(s) ---> Zn2+(aq) + 2eEo = +0.76 V Cu2+(aq) + 2e- ---> Cu(s) Eo = +0.34 V --------------------------------------------------------------Cu2+(aq) + Zn(s) ---> Zn2+(aq) + Cu(s) Eo (calc’d) = +1.10 V Uses of Eo Values • Organize halfreactions by relative ability to act as oxidizing agents • Table 20.1 • Use this to predict cell potentials and direction of redox reactions. 11 TABLE OF STANDARD REDUCTION POTENTIALS oxidizing ability of ion Eo (V) Cu2+ + 2e- Cu +0.34 2 H+ + 2e- H2 0.00 Zn2+ + 2e- Zn -0.76 reducing ability of element 12 13 Potential Ladder for Reduction Half-Reactions Figure 20.11 14 Using Standard Potentials, Eo Table 20.1 • Which is the best oxidizing agent: O2, H2O2, or Cl2? _________________ • Which is the best reducing agent: Hg, Al, or Sn? ____________________ 15 Standard Redox Potentials, Cu2+ + 2e- Cu +0.34 2 H+ + 2e- H2 0.00 Zn2+ + 2e- Zn -0.76 Eo Any substance on the right will reduce any substance higher than it on the left. Northwest-southeast rule: product-favored reactions occur between reducing agent at southeast corner (anode) and oxidizing agent at northwest corner (cathode). 16 Standard Redox Potentials, Eo 17 Any substance on the right will reduce any substance higher than it on the left. • Zn can reduce H+ and Cu2+. • H2 can reduce Cu2+ but not Zn2+ • Cu cannot reduce H+ or Zn2+. Using Standard Potentials, Eo Table 20.1 • In which direction do the following reactions go? • Cu(s) + 2 Ag+(aq) ---> Cu2+(aq) + 2 Ag(s) • 2 Fe2+(aq) + Sn2+(aq) ---> 2 Fe3+(aq) + Sn(s) • What is Eonet for the overall reaction? 18 19 Standard Redox Potentials, Eo E˚net = “distance” from “top” half-reaction (cathode) to “bottom” half-reaction (anode) E˚net = E˚cathode - E˚anode Eonet for Cu/Ag+ reaction = +0.46 V Eo for a Voltaic Cell Cd --> Cd2+ + 2eor Cd2+ + 2e- --> Cd Fe --> Fe2+ + 2eor Fe2+ + 2e- --> Fe All ingredients are present. Which way does reaction proceed? 20 Eo for a Voltaic Cell From the table, you see • Fe is a better reducing agent than Cd • Cd2+ is a better oxidizing agent than Fe2+ Overall reaction Fe + Cd2+ ---> Cd + Fe2+ Eo = E˚cathode - E˚anode = (-0.40 V) - (-0.44 V) = +0.04 V 21 More About Calculating Cell Voltage 22 Assume I- ion can reduce water. 2 H2O + 2e- ---> H2 + 2 OHCathode 2 I- ---> I2 + 2eAnode ------------------------------------------------2 I- + 2 H2O --> I2 + 2 OH- + H2 Assuming reaction occurs as written, E˚net = E˚cathode - E˚anode = (-0.828 V) - (+0.535 V) = -1.363 V Minus E˚ means rxn. occurs in opposite direction 23 Is E˚ related to ∆G? YES! Michael Faraday 1791-1867 Originated the terms anode, cathode, anion, cation, electrode. Discoverer of • electrolysis • magnetic props. of matter • electromagnetic induction • benzene and other organic chemicals Was a popular lecturer. 24 25 o E and o ∆G Eo is related to ∆Go, the free energy change for the reaction. ∆Go = - n F Eo where F = Faraday constant = 9.6485 x 104 J/V•mol and n is the number of moles of electrons transferred Michael Faraday 1791-1867 Eo and ∆Go ∆Go = - n F Eo For a product-favored reaction Reactants ----> Products ∆Go < 0 and so Eo > 0 Eo is positive For a reactant-favored reaction Reactants <---- Products ∆Go > 0 and so Eo < 0 Eo is negative 26 E at Nonstandard Conditions 0.0257 V [Products] E EÞ ln n [Reactants] • The NERNST EQUATION • E = potential under nonstandard conditions • n = no. of electrons exchanged • ln = “natural log” • If [P] and [R] = 1 mol/L, then E = E˚ • If [R] > [P], then E is ______________ than E˚ • If [R] < [P], then E is ______________ than E˚ 27