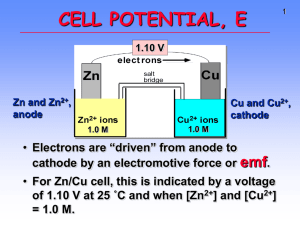
CELL POTENTIAL, E emf
advertisement

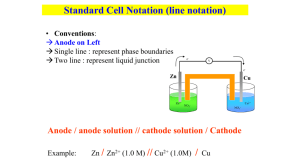
CELL CELL POTENTIAL, POTENTIAL, EE 1 2 CELL CELL POTENTIAL, POTENTIAL, EE Zn and Zn2+, anode • For Zn/Cu Zn/Cu cell, voltage is 1.10 V at 25 ˚C and when [Zn2+] and [Cu2+] = 1.0 M. • This is the STANDARD CELL Cu and Cu2+, cathode • Electrons are “driven” from anode to cathode by an electromotive force or • —a quantitative measure of the tendency of reactants to proceed to products when all are in their standard states at 25 ˚C. • For Zn/Cu Zn/Cu cell, this is indicated by a voltage of 1.10 V at 25 ˚C and when [Zn 2+] and [Cu2+] = 1.0 M. 4 CELL CELL POTENTIALS, POTENTIALS, EEoo Can’t measure 1/2 reaction E o directly. Therefore, measure it relative to a STANDARD HYDROGEN CELL, SHE. 22 H aq, aq, 11 M) atm H++((aq, M) ++ 2e2e- <----> <----> H H22(g, (g, 11 atm) atm)) Eo = 0.0 V Calculating Calculating Cell Cell Voltage Voltage • Balanced half-reactions can be added together to get overall, balanced equation. 2+((aq) Zn(s) (s) ---> aq) Zn aq) ++ 2eZn(s) ---> Zn Zn2+ 2e2+((aq) Cu aq) aq) ++ 2eCu2+ 2e- ---> ---> Cu(s) Cu(s) --------------------------------------------------------------------------------------2+((aq) 2+((aq) Cu aq) (s) ---> aq) aq) ++ Zn(s) Zn aq) ++ Cu(s) Cu2+ Zn(s) ---> Zn Zn2+ Cu(s) POTENTIAL, Eo emf. 3 If we know Eo for each half-reaction, we could get Eo for net reaction. 5 2+ half-cell Zn/Zn Zn/Zn2+ half-cell hooked hooked to to aa SHE. SHE. EEoo for for the the cell cell == +0.76 +0.76 VV Negative electrode Zn Volts - + Positive electrode Salt Bridge 6 Volts Zn - + Salt Bridge H2 Zn2+ Zn Zn2+ + 2eOXIDATION ANODE H+ 2 H+ + 2eH2 REDUCTION CATHODE H2 Supplier of electrons H+ Zn2+ Zn2+ Zn --> 2+ + 2eZn Zn + 2eOxidation OXIDATION Anode ANODE H+ Acceptor of electrons 2 + 2e- --> H2 2 H+ + 2eH Reduction 2 REDUCTION Cathode CATHODE Page 1 Overall reaction is reduction of H + by Zn metal. Zn(s) Zn(s) + 2 H+ (aq (aq)) --> Zn2+ + H2(g) Eo = +0.76 V Therefore, Eo for Zn ---> Zn2+ (aq (aq)) + 2e- is +0.76 V Zn is a (better) (poorer) reducing agent than H 2. 7 2+ and Cu/Cu Cu/Cu2+ and H H22/H /H++ Cell Cell 2+ and Cu/Cu Cu/Cu2+ and H H22/H /H++ Cell Cell 8 Zn/Cu /Cu Electrochemical Zn Zn/Cu Electrochemical Cell Cell wire Volts Eo = +0.34 V Cu + electrons Salt Bridge Positive Cu + - Negative Cu2+ + 2eCu REDUCTION CATHODE Cu2+ Cu2+ + 2e- --> Cu Cu2+ + 2eCu Reduction REDUCTION Cathode CATHODE Supplier of electrons H+ Overall reaction is reduction of by H2 gas. Cu2+ (aq (aq)) + H2(g) ---> Cu(s) + 2 H +(aq) aq) Measured Eo = +0.34 V Therefore, Eo for Cu2+ + 2e- ---> Cu is H --> 2 + 2eH22 Oxidation 2 H+ + 2eOXIDATION Anode ANODE +0.34 V 10 electrons Zn2+ ions salt bridge TABLE TABLE OF OF STANDARD STANDARD REDUCTION REDUCTION POTENTIALS POTENTIALS oxidizing ability of ion wire Zn H2 2 H+ + 2eOXIDATION ANODE Cu 2+ H+ Uses of E Eoo Values • Organize halfreactions by relative ability to act as oxidizing agents • Table 21.1 • Use this to predict cell potentials and direction of redox reactions. Anode, negative, source of electrons H+ Cu2+ Salt Bridge H2 Acceptor of electrons Zn H2 Volts Cu Cu2+ ions Cu2+ + 2e2 H+ + 2eZn2+ + 2e- Eo (V) Cu H2 Zn +0.34 0.00 -0.76 reducing ability of element Page 2 9 salt bridge Zn2+ ions + Cu Cu2+ ions Cathode, positive, sink for electrons Zn(s) Eo = +0.76 V Zn(s) ---> Zn 2+(aq) aq) + 2eCu2+(aq) Eo = +0.34 V aq) + 2e- ---> Cu(s) --------------------------------------------------------------Cu2+(aq) aq) + Zn(s) Zn(s) ---> Zn 2+(aq) aq) + Cu(s) Eo (calc’d (calc’d)) = +1.10 V 11 12 Standard Standard Redox Redox Potentials, Potentials, EEoo oxidizing ability of ion Eo (V) Cu2+ + 2e- Cu +0.34 2 H+ + 2e- H2 0.00 Zn -0.76 Zn2+ + 2e- reducing ability of element Any substance on the right will reduce any substance higher than it on the left. • Zn can reduce H+ and Cu2+. • H2 can reduce Cu 2+ but not Zn2+ • Cu cannot reduce H + or Zn2+. Using Standard Potentials, Eo 13 Table 21.1 EEoo for for aa Voltaic Voltaic Cell Cell EEoo for for aa Voltaic Voltaic Cell Cell Volts • Which is the best oxidizing agent: Cd O2, H2O2, or Cl2? _________________ Fe Salt Bridge Volts • Which is the best reducing agent: Cd Hg, Al, or Sn? ____________________ Cd2+ • In which direction does the following Cd --> Cd2+ + 2eor Cd2+ + 2e- --> Cd reaction go? Cu(s) + 2 Ag +(aq) ---> Cu 2+(aq) + 2 Ag(s) E˚ and G? Fe --> Fe2+ + 2eor Fe2+ + 2e- --> Fe YES! • Balanced half-reactions can be added together to get overall, balanced equation. Fe Salt Bridge Fe2+ 16 Calculating Calculating Cell Cell Voltage Voltage 14 Cd2+ Fe2+ From the table, you see • Fe is a better reducing agent than Cd • Cd2+ is a better oxidizing agent than Fe2+ Overall reaction Fe + Cd2+ ---> Cd + Fe2+ Eo = +0.04 V Michael Michael Faraday Faraday 1791-1867 1791-1867 Originated the terms anode, cathode, anion, cation, cation, electrode. Discoverer of • electrolysis • magnetic props. of matter • electromagnetic induction • benzene and other organic chemicals Was a popular lecturer. 22 II-- ---> ---> II22 ++ 2e2e22 HH22O O ++ 2e2e- ---> ---> 22 OH OH- ++ HH22 ------------------------------------------------------------------------------------------------22 II- ++ 22 HH22O O --> -->II22 ++ 22 OH OH- ++ HH22 If we know Eo for each half-reaction, we could get Eo for net reaction. Page 3 15 18 19 E Eoo and and G G oo Eo is related to Go, the free energy change for the reaction. G o = - n F Eo where F = Faraday constant = 9.6485 x 10 4 J/V•mol J/V•mol and n is the number of moles of Michael Faraday electrons transferred 1791-1867 E Eoo and and G G oo Go = - n F E o For a product-favored reaction Reactants ----> Products Go < 0 and so Eo > 0 Eo is positive For a reactant-favored reaction Reactants <---- Products Go > 0 and so Eo < 0 Eo is negative Page 4 20