Document
advertisement
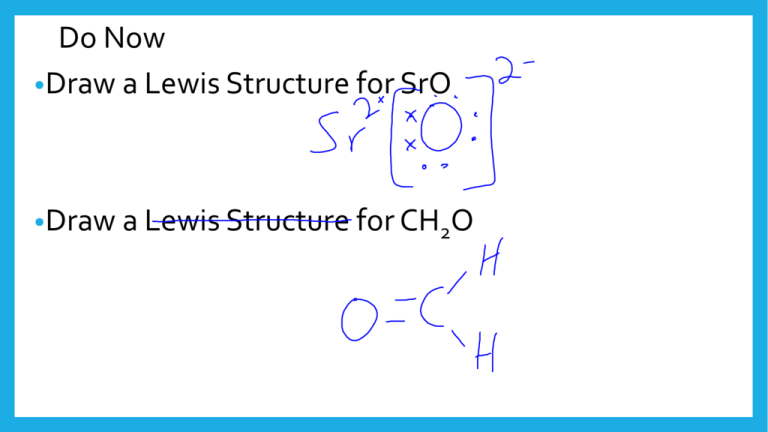
Do Now •Draw a Lewis Structure for SrO •Draw a Lewis Structure for CH2O Aim •How can compounds form both covalent and ionic bonds? Introduction • Draw a Lewis structure of NaCl, then check this molecule for proper octets. • What would it look like if Na and H switched places between the two compounds? Introduction • Pick the best spot to divide these salts into their similar components: A)KCl B) GaH3 C) Al2O3 • Pick the best spot to divide this compound into its similar components: New Idea: Polyatomic ions Regents Review, Round 1 • Which compound contains only ionic bonds? 1. HNO3 2. 3. 4. NH4Cl H2O Na2O Regents Review, Round 2 • Which compound contains both ionic and covalent bonds? 1. HBr 2. CBr4 3. NaBr 4. NaOH Regents Review, Round 3 • Which is the formula for sodium perchlorate? 1. NaClO 2. NaClO2 3. NaClO3 4. NaClO4 Regents Review, Round 4 • Which compound contains both ionic and covalent bonds? 1. Ammonia 2. Methane 3. Sodium nitrate 4. Potassium chloride Regents Review, Round 5 • Which compound contains both ionic and covalent bonds? 1. CaCO3 2. PCl3 3. MgF2 4. CH2O









