HL STANDARD E
advertisement

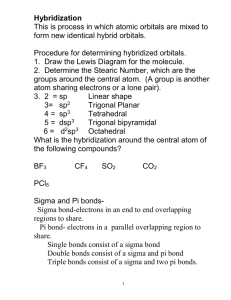
LEWIS STRUCTURES MOLCULAR GEOMETRY & HYDRIDIZATION WHEN THE CENTRAL ATOMS IS AN ELEMENT FROM THE 3rd PERIOD OR BELOW, WE SOMETIMES FIND COMPOUNDS IN WHICH THERE ARE MORE THAN EIGHT ELECTRONS AROUND THE CENTRAL ATOM. THIS ARRANGEMENT IS POSSIBLE BECAUSE THE d ORBITALS IN THE VALANCE SHELL OF THESE ATOMS HAVE ENERGY VALUES CLOSE TO THOSE OF THE p ORBITALS TYPES OF COVALENT BONDS SINGLE BONDS •LONGEST OF THE 3 TYPES •WEAKEST OF THE 3 TYPES •CONTAINS ONE PAIR OF ELECTRONS (2 ELECTRONS) DOUBLE BONDS •LENGTH IS GREATER THAN TRIPLE BUT LESS THAN SINGLE •STRENGTH IS GREATER THAN SINGLE BUT LESS THAN TRIPLE •CONTAINS TWO PAIRS OF ELECTRONS (4 ELECTRONS) TRIPLE BONDS •SHORTEST OF THE 3 TYPES •STRONGEST OF THE 3 TYPES •CONTAINS THREE PAIRS OF ELECTRONS (6 ELECTRONS) LONGEST BONDS(SINGLE) ARE THE WEAKEST SHORTEST BONDS(TRIPLE) ARE THE STRONGEST VSERP THEORY states that electron pairs found in the outer energy level or valence shell of atoms repel each other and thus position themselves as far apart as possible The VSERP theory helps to predict the shape of the molecule. The following points should be considered in predicting shape (molecular geometry): lone-lone pair>lone-bonding pair>bonding-bonding pair FOR EXAMPLE, IN THE LEWIS STRUCTURE OF WATER, THERE ARE TWO NONBONDED (LONE) PAIRS OF ELECTRONS ON THE OXYGEN ATOM. THUS THE NON-BONDED (LONE) PAIR OF ELECTRONS REPEL MORE THAN THE LONEBONDED PAIR BETWEEN THE OXYGEN AND HYDROGEN AND THE BONDEDBONDED PAIR OF ELECTRONS BETWEEN THE TWO HYDROGENS HAVE THE LEAST REPULSION. NON-BONDED (LONE)& NON-BONDED (LONE) PAIR OF ELECTRONS NON-BONDED(LONE) & BONDED PAIR OF ELECTRONS BONDEDBONDED PAIR OF ELECTRONS Due to the fact that lone pair of electrons repel more than bonded pairs, the bond angles will be different in different shaped molecules BEND(with 2 lone pair of e-) <TRIGONAL PYRAMIDAL<TETRAHEDRAL< BEND(with 1 lone pair of e-)<TRIGONAL PLANAR<LINEAR ****KNOW THE BOND ANGLES OF THIS SHAPES TETRAHEDRAL 109.5o TRIGONAL PLANAR LINEAR 180o 120o .. S // \ :O: :O: .. BENT (w/ TRIGONAL PYRAMIDAL 109.5o (107.5o ) 1 lone pairs of e-) 120o (119o) BENT (w/ 2 lone pairs of e-) 109.5 o (104.5o) ETHANOIC ACID CH3COOH BENT 104.5o TETRAHEDRAL TRIGONAL PLANAR CAN A MOLECULE BE NONPOLAR AND STILL HAVE POLAR BONDS WITHIN THE MOLECULE??? FIRST THINK ABOUT WHAT MAKES A BOND POLAR! NOW THINK ABOUT WHAT MAKES A MOLECULE NONPOLAR! YES THE DIFFERENCE IN ELECTRONEGATIVITY VALUES (CHARGE SEPARATION) DETERMINE IF A BOND WILL BE POLAR OR NONPOLAR. 0-.3 difference is NONPOLAR .3-1.7 difference is POLAR IF A MOLECULE IS SYMMETRICAL OR IF THERE IS NO NET DIPOLE MOMENT THEN IT IS NONPOLAR EXAMPLE: CCl4 THE C-Cl BOND IS POLAR BUT THE MOLECULE IS TETRAHEDRAL SO IT IS SYMMETRICAL AND THUS NONPOLAR WHICH OF THE FOLLOWING BONDS IS THE MOST POLAR B-C or C-O or N-O or O-F ANSWER: C-O b/c they are further apart on the periodic table which means that they have the greatest charge separation. HYBRIDIZATION IS THE MIXING OF ORBITALS THE NEW HYBRID ORBITALS FORMED MAY BE sp, sp2, sp3, (sp3d, sp3d2 not covered) Formation of sp hybrid orbitals The combination of an s orbital and a p orbital produces 2 new orbitals called sp orbitals. These new orbitals are called hybrid orbitals The process is called hybridization What this means is that both the s and one p orbital are involved in bonding to the connecting atoms EXAMPLES OF MOLECULES WITH sp HYBRIDIZATION ALL LINEAR MOLECULES Formation of sp2 hybrid orbitals EXAMPLES OF sp2 HYBRIDIZATION ALL TRIGONAL PLANAR AND BENT(one lone pair of e-) .. S // \ :O: :O: .. Formation of sp3 hybrid orbitals EXAMPLES OF sp3 HYBRIDIZATION ALL TETRAHEDRAL TRIGONAL PYRIMIDAL & BENT (w/ two lone pair of e-) Hybrid orbitals can be used to explain bonding and molecular geometry Multiple Bonds Everything we have talked about so far has only dealt with what we call sigma bonds Sigma bond (s) A bond where the line of electron density is concentrated symmetrically along the line connecting the two atoms. Pi bond (p) A bond where the overlapping regions exist above and below the internuclear axis (with a nodal plane along the internuclear axis). Example: H2C=CH2 Example: H2C=CH2 Example: HCCH Delocalized p bonds When a molecule has two or more resonance structures, the pi electrons can be delocalized over all the atoms that have pi bond overlap. Example: C6H6 benzene Benzene is an excellent example. For benzene the p orbitals all overlap leading to a very delocalized electron system In general delocalized p bonding is present in all molecules where we can draw resonance structures with the multiple bonds located in different places.