chem32A Lab quiz3
advertisement

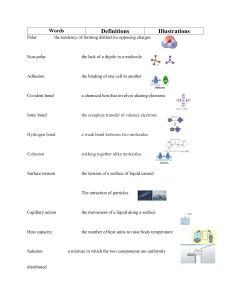
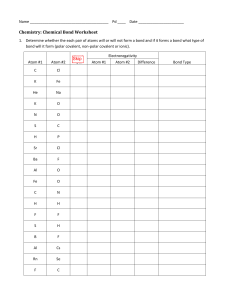
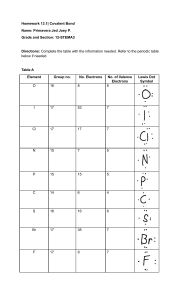
SJCC Chem. 32A Prof. Song Quiz #3 (a) Name: 1. Name or give chemical formula of the following compounds: (4 pt) (a) HClO3(aq) (b) H3PO4(aq) (c) Hydroiodic acid (d) sodium phosphate 2. Classify the following compounds as ionic or covalent. (4 pt) (a) Calcium chloride, CaCl2 (c) Sucrose, C12H22O11 3. Draw Lewis dot structure and predict the three-dimensional molecular geometry around the central atom for each: (8 pt) Lewis structure (show all bonding and nonbonding electrons) Molecular structure (linear, trigonal planar, trigonal pyramid, tetrahedral) CH4 NH3 Circle the best answer for the following: (4 pt) 4. In which of the following cases is a polar covalent bond formed? (a) (b) (c) (b) when an electron is transferred from one atom to another when the electrons of a bond are shared equally by the two atoms when the electrons of a bond are shared unequally by the two atoms when a metallic element forms a bond with a non-metallic element