Vision + Desensitization
advertisement

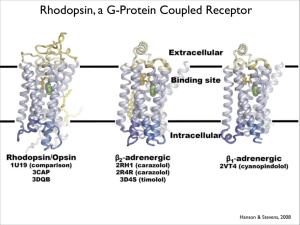
OUR 5 MAJOR SENSORY SYSTEMS Vision - the detection of light Olfaction- (sense of smell) the detection of small molecules in the air Taste or Gustation- the detection of selected organic compounds and ions by the tongue Hearing-The detection of sound (or pressure wave in the air) Touch- the detection of changes in pressure, temp. and other factors by the skin SENSORY SYSTEMS When fully adapted to darkness our eyes allow us to sense very low levels of light, down to a limit of less than 10 photons. With more light we are able to distinguish millions of colors. Through our senses of smell and taste we are able to detect thousands of chemicals and sort them into distinct categories Each of these primary sensory systems contains specialized sensory neurons that transmit nerve impulses to the CNS In the CNS theses signals are processed and combined with other information to yield a perception that may trigger a change in behavior. By these means, our senses allow us to detect changes in our environments and adjust our behavior appropriately Photoreceptor molecules in the eye detect visible light Vision is based on the absorption of light by photoreceptor cells in the eye Photoreceptor cells are sensitive to light in a relatively narrow region of the electromagnetic spectrum between 300-850nm Two kinds of photoreceptors Rods (100 million) and Cons (3 million) Rods function in dim light and do not perceive color Cons function in bright light and are responsible for color vision VISION Pigment epithelium Neuronal layers The Retina • Contains photoreceptor cells (rods and cones) and associated interneurones and sensory neurones light t oopt c i gango il n nerve cels bp i oa lr neurones rod conepigm ent ed cels cels ret ina Vision---rod/cones The neural circuits in the retina of a primate -The incoming light reaches the photoreceptor cells (rods and cones) only after passing through several thin, transparent layers of other neurons. -The pigment epithelium absorbs the light that is not absorbed by the photoreceptor cells and thus minimizes reflections of stray light. The ganglion cells communicate to the thalamus by sending action potentials down their axons. However, the photoreceptor cells and other neurons communicate by graded synaptic potentials that are conducted electronically. The Rod Cell Scanning electron micrographs of retinal rod cells Schematic representation of a rod cell 100,000,000 rod cells in human retina Photoperception 1000 disks, 16nm thick Rod cell (1x40µm) Biochemistry. L. Stryer The disks which are membrane enclosed sacs are densely packed with photoreceptor molecules The photosensitive molecule is called the visual pigment because it is highly colored due to light absorption The photoreceptor molecule in the rods is rhodopsin consists of opsin linked to 11-cis-retinal 300-850nm The electromagnetic spectrum Absorption spectrum of rhodopsin Questions How does the cell respond to photons? What mechanism converts light into a cellular signal? Ligand-activated Receptor Light-activated Receptor Rhodopsin (polyene- with 6 alternating double and single bonds) Illustration of Rhodopsin (blue) with 11-cis retinal (red) (440nm absorption) The protonated form of the 11-cis retinal absorbs at 440nm Unlike 380nm of the non-protonated. The positive charge of Lys296(VII) is compensated by Glu113(II) Activation of rhodopsin by a photon-converting a light energy of A photon into atomic motion -The isomerization causes the Shiff-base nitrogen to move approximately 5A, assuming that the cyclohexane ring of the cis-retinal group remains fixed/ -Inverse agonist- 108 Rhodopsin molecules /cell RHODOPSIN In Glu113 D(E)RY Cys322 Cys323 Helix VIII (311-321) Asp2 Out Cys 110 Cys 187 Met1 Lys 296 Glu181 Asp15 The three dimensional structure of rhodopsin Rhodopsin 2.8A resolution; Science 289, 739-745 (2000) Science 389,739 (2000) Three dimensional Model of Rhodopsin Palmitoyl at Helix 8 Retinal Rhodopsin photoactivation Alcohol dehydrogenases Transducin at 39kD; b 36kD; 8kD a b In the dark transducin is in the GDP form the binding of GTP to transducin leads to the release of R* which enables it to catalyze the Activation of another molecule of transducin A single R* catalyzes the activation of 500 molecules of transducin, the first stage in the amplification of vision Schematic diagram of the cyclic GMP cascade of vision Activation of phosphodiesterase by Gat The binding of GTP switches on the phosphodiesterase (PDE) by relieving an inhibitory constraint. In the dark the two catalytic subunits a and b are held in check by a pair of inhibitory subunits (). By binding of Gat to the enzyme it removes the inhibitory subunits and the enzyme is activated a Inactive b Gat Gat a b Gat Active The hydrolysis of cGMP by phosphodiesterase is the second stage of of amplification 11-cis-retinal 11-trans-retinal Membrane potential Light hyperpolarizes the plasma membrane of a retinal rod cell The light induced hyperpolarization is transmitted by the plasma membrane from the outer segment to the synaptic body. A single photon closes hundreds of cation specific channels (~500) and leads to a hyperpolarization of about 1-5mV Cation channels (~500) in the rod cell close following the transduction of a single photon. These represent 3% of the total number of channels that are open in the dark. The resultant hyperpolarization is about 1mV and lasts about 1 sec. This is sufficient to depress the rate of neurotransmitter release that transmits the onward signal The high-degree of co-operativity (3 molecules of cGMP) to open the channel increases the sensitivity of the channel for small changes in cGMP which enable it to act as a switch. CNG- Cyclic nucleotide-gated channels Cyclic nucleotide binding domain Dark Current BiologyMad.com In the Dark… • In the dark the channel is open Na+ flow in can cause rod cells to depolarise. – Therefore in total darkness, the membrane of a rod cell is polarised • Therefore rod cells release neurotransmitter in the dark • However the synapse with bipolar cells is an inhibitory synapse i.e. the neurotransmitter stops impulse BiologyMad.com BiologyMad.com In the Light… As cis retinal is converted to trans retinal, the Na+ channels begin to close i less neurotransmitter is produced. If the threshold is reached, the bipolar cell will be depolarised i forms an impulse which is then passed to the ganglion cells and then to the brain BiologyMad.com BiologyMad.com Rods and Cones Rods Cones Outer segment is rod shaped 109 cells per eye, distributed throughout the retina, so used for peripheral vision. Good sensitivity Only 1 type monochromatic vision Outer segment is cons shaped 106 cells per eye, found mainly in the fovea, so can only detect images in centre of retina. Poor sensitivity 3 types (R, G & B) colour vision Many rods connected to one bipolar cell poor acuity = poor resolution Each cone is connected to one bipolar cell good acuity = good resolution One rhodopsin molecule Absorbs one photon 500 Transducin molecules are activated 500 Phospodiesterase molecules are activated 105 cGMP molecules are hydrolyzed 250 Na+ channels closed 106-107 ions/sec are prevented from entering the cell for a period of 1 sec Rod cell membrane is hyperpolarized by 1 mV Guanylate cyclase GTP cGMP +PPi The enzyme Guanylate cyclase looses its activity in high Ca2+ BiologyMad.com Color Vision • 3 different cone cells. Each have a different form of opsin (they have the same retinal) • 3 forms of rhodopsin are sensitive to different parts of the spectrum – 10% red cones – 45% blue cones – 45% blue cones Con Cells The absorption spectra of the cone visual pigment responsible for color vision The cone photoreceptors are 7TM domain receptors that utilize 11-cis-retinal as chromophore. Absorption maxima (nm) in human are 426 (blue), 530 (green) and 560 (red) Comparison of the amino acid sequence of the green and red photoreceptors Color Vision • Colored light will stimulate these 3 cells differently - by comparing the nerve impulses from the 3 kinds of cones the brain can detect any colour – – – – Red light stimulates R cones Yellow light stimulates R and G cones equally Cyan light stimulates B and G cones equally White light stimulates all 3 cones equally • Called the trichromatic theory of color vision Color Vision • When we look at something the image falls on the fovea and we see it in color and sharp detail. • Objects in the periphery of our field of view are not seen in colour, or detail. • The fovea has high density of cones. • Each cone has a synapse with one bipolar cell and one ganglion each cone sends impulses to the brain about its own small area of the retina high visual acuity Evolutionary relationships among visual pigments Visual pigments have evolved by gene duplication Color blindness The genes for the green and red pigments lie adjacent on the human X chromosome. Are 98% identical in nucleotide sequence including introns and UTR -Therefore, are susceptible for to unequal homologous recombination -5% of males have this form of blindness Recombination pathways leading to color blindness Rearrangements in the course of DNA replication A) Loss of visual pigment B) The formation of hybrid pigemnt genes that encode photoreceptors with anomalous abs. spectra A homologous recombination: the exchange of DNA segment at equivalent positions between chromosomes with substantial similarity Termination of the signal One of the most important part of the signaling machinery is termination of the signal even in the presence of the stimulus This phenomenon is referred to as “desensitization” Such mechanisms operate at both the level of the receptor as well as down stream at the level of G-protein Rapid termination of the receptor signal is controlled by receptor phosphorylation which is mediated by second messenger-kinases PKA and PKC or by a distinct Receptor-kinsases (GRKs) together with arrestins Heterologous desensitization Second-messenger kinase regulation PKA and PKC uncouple receptors from their respective G-proteins and serve as negative-feedback regulatory loops. Feed back regulation by the 2nd messengerstimulated kinases PKA and PKC. The phosphorylated receptor changes its conformation and no longer can activate the Gproteins. It is an agonist non-specific desensitization Homologous desensitization GRK(G-ptrotein-receptor kinase)-mediated desensitization A complex mechanism for regulating 7TM-receptor activity called GRK-barrestin system It is also called an agonist-specific desensitization because only the activated agonist-occupied conformation of the receptor is phosphorylated by by GRK. A two step process in which agonist-occupied receptor is phosphorylated by GRK and then binds an arrestin proteins. This leads to a rapid-agonist specific desensitization Heterogous and homologous desensitization The major GPCR regulatory pathway involves phosphorylation of activated receptors by G protein–coupled receptor kinases (GRKs), followed by binding of arrestin proteins, which 1) prevent receptors from activating downstream heterotrimeric G protein pathways while 2) allowing activation of arrestin-dependent signaling pathways. GRK - G-protein–coupled receptor kinase As long as the agonist remains bound to the receptor, the activated receptor can continue to activate G proteins. GRK which is catalytically activated by this interaction, also recognizes the activated conformation of the receptor. Activated GRKs phosphorylate (P) intracellular domains of the receptor and are then released. The agonist-activated, GRKphosphorylated receptor binds tightly to an arrestin protein, which desensitizes further G protein activation and couples the receptor to the clathrin-coated-pit internalization pathway and to arrestinscaffolded (and G protein–independent) signaling pathways. GRK-GPCR-kinase The role of GRK-phosphorylation of the receptors in the sequestration process is to facilitate arrestin binding Experiments to prove this idea 1)A mutated b-adrenergic receptor Y326A is a poor substrate for b-Adrenergic receptor-kinase, and is not sequestered. Over-expression of b-arrestin restores sequestration 2) Removal of C-terminal tail (sites for GRK sites) prevents sequestration Arrestins The arrestin family includes > 6 members several of which undergo alternative splicing The affinity of b-arrestin (selective for the breceptors) increases 10-30 fold by GRK-catalyzed phosphorylation, whereas agonist occupancy has a much less significant effect. The b-arrestins promote internalization by binding to clatherin Science, 297, 529 (2002) badrenergic receptor PKA PKA Homologous desensitization bark Rhodopsin Rhodopsin kinase Termination Activation GTP RGS and GAP Activities GDP GTP GDP Neuron 20, 11-14 (1999) 11-cis vs. all-trans retinal