lecture four slides

mammalian eyes
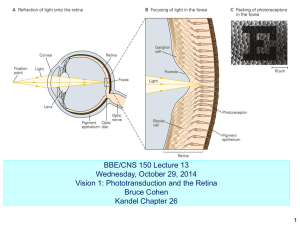
image formation
vertebrate retina 1
• receptors at the back, embedded in the pigment epithelium, nerves and blood vessels at the front, nearest the lens
• receptors are depolarised in the dark, secreting glutamate
• receptors synapse with bipolar cells and horizontal cells
• there are at least eleven types of bipolar cells, four of which are specific either for rods or for a particular type of cone, others work with multiple receptors and horizontal cells to integrate information from a wider area
• there are two major classes of bipolar cells, which differ in their glutamate receptors:
– OFF centre channels hyperpolarise in response to light, similar to the photoreceptors
– ON centre channels depolarise in response to light, opposite to the photoreceptors
vertebrate retina 2
• most cells in the retina are analogue machines that respond to light with graded changes in membrane potential
• horizontal cells and amacrine cells help to integrate responses over a wider area
• the two main types of horizontal cells respond either to the background intensity or to its colour
• ganglion cells are the first cells in the visual pathway to fire action potentials in response to stimulation
• there are ~25 different types of amacrine cell and a similar variety of ganglion cells, which encode complex features of the retinal image
• most ganglion cells serve central regions of the retina, but at the periphery several receptors share each ganglion cell
visual acuity
• there are no photo-receptors in the blind spot, where the blood vessels and optic nerve enter the eyeball
• visual acuity is much higher in the centre of the field, in the macula and especially in the fovea
• rods provide slow monochrome vision in dim light
• rods are much more common at the periphery of the retina
• three types of cones (in primates) provide high-speed, high definition colour vision in stronger light
• cones are densely packed in the macula, and especially in the fovea, which is entirely free from rods
• most mammals are dichromats with only two types of cone, but fish, reptiles and birds may be tetra- or penta-chromats
ribbon synapses
• In conventional nerve synapses, the fusion of synaptic vesicles with the pre-synaptic membrane and release of neurotransmitter is intermittent, triggered by individual action potentials.
• Vertebrate photoreceptors , retinal bipolar cells and hair cells in the inner ear [next lecture] are analogue transducers which secrete glutamate continuously, in proportion to graded changes in membrane potential.
• This requires a special type of “ribbon synapse” which is specially adapted for continuous rapid neurotransmitter release.
• The ribbon tethers synaptic vesicles and supplies them in a steady stream to the synaptic junction.
Joselevitch & Zenisek (2010) Neuron 68, 604-606
some papers on ribbon synapses
• Sterling & Matthews (2005) Structure and function of ribbon synapses.
TRENDS in Neurosciences 28 , 20-29.
• Schmitz (2009) The Making of Synaptic Ribbons: How
They Are Built and What They Do.
Neuroscientist 15 ,
611-624.
• Joselevitch & Zenisek (2010) The Cytomatrix Protein
Bassoon Contributes to Fast Transmission at
Conventional and Ribbon Synapses.
Neuron 68 , 604-
606.
• Matthews & Fuchs (2010) The diverse roles of ribbon synapses in sensory neurotransmission.
Nature Reviews in Neuroscience 11 , 812-822.
Schmitz (2009) Neuroscientist 15, 611-624.
Synapse between a photoreceptor cell, horizontal cells (hc) and a bipolar cell (bc). The fan-shaped synaptic ribbon guides the synaptic vesicles towards the pre-synaptic membrane.
Schmitz (2009) Neuroscientist 15, 611-624.
hugely modified primary cilia!
photo-receptors
• photo-transduction in almost all species (except C. elegans ) uses rhodopsin, which is an integral membrane protein
• vertebrate photoreceptors are hugely modified primary cilia , which are embedded in the retinal pigment epithelium
• the membrane is thrown into folds or discs, increasing area
• a new fold is generated every 20 minutes, and takes about one month to travel down a rod outer segment, before it is shed and phagocytosed by the retinal pigment epithelium
• rods provide monochrome vision under low light conditions
• three types of cones provide colour vision in stronger light, and are concentrated in the high acuity macula & fovea
• spectral sensitivity depends on the opsin protein sequence
rods & cones
optic nerve
• The optic nerve relays via the lateral geniculate nucleus and the superior colliculus to map specific locations in the retina to corresponding locations in the visual cortex
• Visual signals are highly compressed digital information, which allows the optic nerve to be much thinner and more flexible than a simple analogue connection
• Ganglion cells can transmit the centre-ON and centre-OFF patterns from the bipolar cells, but also encode a variety of sum and difference signals, for example green spots on red backgrounds.
• Specialist ganglion cells report movement and orientation.
Such information is biologically relevant to the organism.
visual transduction mechanism
visual transduction mechanism
vertebrate photo-transduction mechanism
• cis -retinal binds to opsin, creates rhodopsin photo-pigment
• receptors are depolarised in the dark, secreting glutamate
• light converts cis -retinal to trans -retinal, activates rhodopsin
• photoactivated rhodopsin repeatedly activates hundreds of molecules of the G-protein transducin – amplifier 1
• transducin a subunits temporarily activate 3’5’cyclic GMP phosphodiesterase enzyme (until the bound GTP is split)
• activated phosphodiesterase splits thousands of molecules of 3’5’cyclic GMP per second – amplifier 2
• reduction in c-GMP closes plasmalemma sodium channels, leads to a fall in cytosolic sodium and calcium ions
• receptor cells hyperpolarise, stop secreting glutamate
vertebrate adaptation & recovery
• our eyes use multiple systems to adapt to 1,000,000,000 fold changes in light intensity
• the 10-fold fall in cytosolic calcium during photo-activation activates rhodopsin kinase and cyclic GMP synthesis
• rhodopsin kinase phosphorylates activated rhodopsin, after which arrestin binds to terminate rhodopsin action
• trans -retinal dissociates from opsin, and is slowly recycled back to cis -retinal by the retinal pigment epithelium
• lack of cis -retinal leads to receptor bleaching, this reduces cone sensitivity and completely abolishes rod transduction at normal daytime light intensities
• transducin, arrestin and recoverin move between the inner and outer segments as part of the adaptation process
protein translocation
• Transducin – rhodopsin-sensitive G protein which activates c-GMP phosphodiesterase.
• Rhodopsin kinase – phosphorylates activated rhodopsin so that arrestin can bind to it.
• Arrestin – binds to phosphorylated rhodopsin and blocks transducin activation.
• RGS9 – regulator of G-protein signalling, which speeds up
GTP hydrolysis, terminating transducin action.
• Recoverin – undergoes a conformational change after calcium binding to expose a myristoyl group, sticks to disk membranes and inhibits rhodopsin kinase. Recoverin is more active in the dark when calcium levels are higher.
protein translocation during adaptation
Lobanova et al (2010) Mechanistic Basis for the Failure of
Cone Transducin to Translocate: Why Cones Are Never
Blinded by Light.
J. Neuroscience 30 , 6815 – 6824.
• Translocation of regulatory proteins between subcellular compartments is much more important for adaptation in rods than it is in cones.
• Knockout of cone RGS9 increases the translocation of cone transducin in response to light.
• Expression of rod rhodopsin in cones increases the translocation of transducin in response to light.
• These investigators conclude that the half-life of photoactivated rod rhodopsin is much longer than that of cone rhodopsin, so more free transducin is generated and is therefore available for translocation within the cell.
Lobanova et al (2010) J. Neurosci.
30
, 6815 – 6824.
invertebrate vision
Drosophila head:
Gordon Beakes
© University of
Newcastle upon Tyne
invertebrate transducers
• Some simple metazoans (including C. elegans ) lack opsin but contain other light-sensitive proteins. Such species have no image-forming abilities, but shun the light to avoid desiccation or predators.
• Phototransducers come in numerous shapes and sizes, and many species have more than one variety
• The ancestral opsin gene has evolved (with its various regulatory proteins) to generate two families of photoreceptors:
– ‘ciliary’ sensors common in deuterostomes (including mammals) are hugely modified cilia containing c-opsin
– ‘ rhabdomeric’ sensors common in protostomes (including insects) are modified microvilli containing r-opsin
• The simplest animals with eyes are cnidarians (hydra, sea anemonies & jellyfish) which use the ‘ciliary’ gene set.
• The primitive chordate amphioxus has eyes containing both types of receptor.
High Resolution Imaging Facility
University of Alabama
Tomasz Szul
Dros ophil a
Compound Eye
• 750 ommatidia per eye
• each ommatidium has:
• 8 photoreceptor cells
• 4 cone cells
• 2 primary pigment cells
• 6 shared pigment cells
• non-cellular chitin lens
• From “The Interactive Fly” hosted by the Society for
Developmental Biology http://www.sdbonline.org/fly/ aimain/1aahome.htm
• Drawing © Donald Ready
• Wolff, T. and Ready, D. F.
(1993). Pattern formation in the Drosophila retina.
In:
The Development of
Drosophila melanogaster.
Cold Spring Harbor
Laboratory Press.
Vol. 2 Pp. 1277-1325
horses for courses
vertebrates
• Mainly ciliary receptors
• Simple steerable eyes
• Higher foveal resolution
• Colour vision
• Variable focus
• Stereoscopic option
• Metabotropic system
• Single photon detection
• Wide dynamic range
• Best for image analysis insects
• Rhabdomeric receptors
• Fixed compound eyes
• Lower resolution
• Monochrome vision
• Fixed focus
• Unsuitable for stereo
• Metabotropic system
• Single photon detection
• Wide dynamic range
• Best for speed
vertebrates drosophila photoreceptor rod & cones (modified cilia) rhabdomeres (microvilli) dark condition initial effect of light retinal dissociation?
retinal recycling depolarised, secreting glutamate meta-rhodopsin II activates
G protein (transducin) opsin and dissociate trans -retinal must slow, involves pigment cells
G protein releases free Gα t subunits polarised, not secreting meta-rhodopsin II activates G protein no obligatory dissociation, but see Arshavsky (2010) below pigment cells, plus fast route to reform cis -retinal using red light releases free Gα q subunits phospholipase C β4 target enzyme cGMP phosphodiesterase response terminator second messenger
RGS-9 + PDEγ regulate GTPase activity cGMP falls in light
RGS(?) + PLC regulate GTPase activity diacylglycerol rises in light
vertebrates plasmalemma effect of light adaptation 1 adaptation 2 cGMP-gated Na + / Ca ++ TRP channels close receptor hyperpolarises, stops secreting
Ca ++ falls; guanyl cyclase activity rises rhodopsin kinase phosphorylates opsin drosophila
DAG-gated TRP channels open receptor depolarises, starts secreting
Ca ++ rises; +ve feedback within microvillus rhodopsin kinase phosphorylates opsin adaptation 3 arrestin inactivates rhodopsin arrestin inactivates rhodopsin adaptation 4 adaptation 5 adaptation 6 translocate proteins between compartments translocate proteins between compartments switch from rods to less sensitive cones close pupil all rhabdomeres have similar sensitivities
Ca ++ -dependent movement of pigment granules
Arshavsky (2010) Vision: The Retinoid Cycle in
Drosophila.
Current Biology
20
, R96-R98
It was previously believed that vertebrates recycled trans retinal via the pigment epithelium, but insects relied on in situ re-isomerisation catalysed by orange light.
Recent results suggest that insects also rely on pigment cells for part of this task.
evolution of eyes
• Erclik et al (2009) Developmental Biology 332 , 70 – 79.
• Lamb (2009) Phil Trans Roy Soc B 364 , 2911 – 2924.
• Plachetzki et al (2010) Proc. R. Soc. B 277 , 1963 –1969.
• Many types of eye appear suddenly in the fossil record 543 million years ago across multiple phyla, and rapidly approach their modern forms.
• Vertebrates mainly use primary cilia , insects use microvilli .
• Vertebrates respond to light off , insects respond to light on .
• BUT all species use Pax6 genes to control eye development, and vertebrates have “insect” melanopsin in ganglion cells.
• Choanoflagellates lack opsin, but have a cilium surrounded by microvilli
• Cnidaria (hydra, jellyfish) have opsin linked to CNG channels.
• Primitive organisms may have used BOTH mechanisms to detect sunrise and sunset over 600 million years ago, before image formation evolved.
adapted from
Plachetzki et al (2010) Proc. Roy. Soc. B 277 , 1963 –1969