Worksheet Thermochemistry I Answers
advertisement

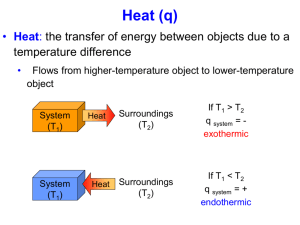
Science 10 Brakke/Baier/Heathcote Worksheet Thermochemistry I – Answer Key Take the following notes from the powerpoint: What is the difference between temperature and thermal energy? ECA – Chemistry – Topic 01 What is a calorie? What is a joule? What is Calorimetry? What is the difference between the system and the surroundings? What is the difference between endothermic and exothermic? The formulas (equations) for heat change are? (one uses ms, the other, c) Water is a very common substance used in calorimetry, what is the specific heat of water as a liquid? Where: q= s= c= m= ΔT = Example: Follow the example in the powerpoint below: How much heat is given off when an 869 g iron bar cools from 94 0C to 50C? The specific heat of Fe is 0.444 J/g • 0C What is enthalpy? Draw an example of an endothermic and an exothermic process, include the equations and the value for ΔH: Exothermic Endothermic Problems: Show ALL work for these problems. Show a data table, the formula you are using, substituting into the formula, with UNITS and then the answer. No work = grade of 0. Science 10 Brakke/Baier/Heathcote ECA – Chemistry – Topic 01 1. Calculate how much energy it takes to raise the temperature of 125 grams of aluminum from 100 to 245 degrees C. The specific heat of aluminum is 0.900 J/g degree C q=? m=125g Al s = 0.900 J/goc ΔT = +145 oc 2. Calculate the heat change needed to raise the temperature of 1000. g of water from 5.0 degrees C to 95.0 degrees C. q=? m = 1000 g H2O s = 4.184 J/goc ΔT = +90.0oc 3. q = msΔT 10500 J = (520 g)( s )(218oc) s = 0.0926 J/goc Determine the specific heat of an unknown element if it takes 540 J to raise the temperature of 55.6 grams of this element from 5.0 to 95.0 degrees C. q = + 540 J m = 55.6.g Cu s = _______ J/goc ΔT = + 90.0oc 7. q = msΔT q = (1000 g)(4.184 J/goc)(-90.0oc) q = - 376,560 J q = - 377 kJ (exothermic) Determine the specific heat of copper if it takes 10 500 J of energy to raise 520.0 grams of it from 27.0 degrees C to 245.0 degrees C. q = + 10,500 J m = 520.g Cu s = _______ J/goc ΔT = + 218oc 6. q = msΔT q = (1000g)(4.184 J/goc)(90.0oc) q = + 376,560 J q = + 377 kJ (endothermic) How much energy is given off by the water in question number two if the 1000. g of water cools from 95.0 to 5.0 degrees C? q=? m = 1000 g Al s = 4.184 J/goc ΔT = -90.0oc 4. q=msΔT q=(125g)(0.900 J/goc)(145oc) q = 16,312.5 J q = 16.3 kJ (endothermic) q = msΔT 540 J = (55.6 g)( s )(90oc) s = 0.1079 J/goc If a reaction (chemical or physical) takes in heat energy we say it is ……endothermic……….., and the sign of q is …( + )…. If a reaction gives (chemical or physical) gives off heat energy we say it is …exothermic……., and the sign of q is …( - )…..