Changes in Matter and Energy
advertisement

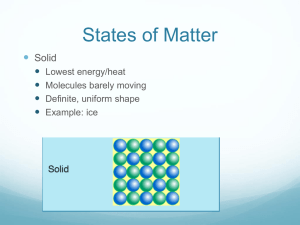
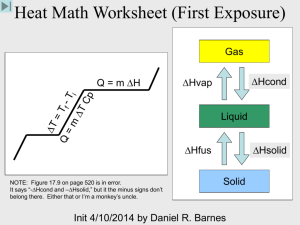
Changes in Matter and Energy • Matter cannot be created or destroyed. • But it can be changed and when it does, that is how we get energy! • Energy - capacity to do work or produce heat – Energy is always involved in physical and chemical changes. – Energy can take several forms: Heat, light, (sound, chemical, electrical) – Measured in calories, Calories (kcal), and joules • Law of Conservation of Energy: Energy cannot be created or destroyed. • Energy can be transferred. Kinetic and Potential Energy Kinetic energy: is the energy of motion. Potential Energy: energy of position; stored energy CHEMICAL BONDS!!! • Total Energy = KE + PE • Temperature: kinetic energy of all particles within matter. • Total Energy for a “system” doesn’t change Endothermic – energy is absorbed – Substance gets cold – Loss of KE, gain of PE – Ex: Class demo Exothermic – energy is released – Substance gets hot – Gain of KE, loss of PE, more KE – Ex: gummy bear sacrifice Exothermic or Endothermic? • • • • Releases energy Temperature rises Gets colder Needs energy to be added for the reaction to occur • Chemicals have less potential energy after the reaction • Chemicals have more potential energy after the reaction Create a Venn Diagram for Exothermic and Endothermic Reactions Energy Specific Heat (s) : amount of energy (heat) required to change the temperature of one gram of a substance by 1 oC Varies from one substance to another Calorie (cal): the amount of energy required to raise the temperature of one gram of water by one Celcius degree Standard unit for energy is the joule (J) 1 cal = 4.184 J • Water has a specific heat = 1 cal/goC or 4.184 J/goC – Water has the second highest specific heat capacity of all known substances. So it requires high amounts of heat energy to raise water temperature. – water also has a high energy/heat requirement for evaporation • SIRON = 0.449 J/goC – Which would heat up faster, 5.00 grams of iron or 5.00 grams of water? – Which would cool down faster, 5.00 grams of iron or 5.00 grams of water? – Which is a better thermal conductor? – Which is a better insulator? Q = s x m x DT Q = energy (heat) required (J) or (cal) s = specific heat capacity (J/goC) or (cal/goC) m = mass of the sample in grams DT = change in temperature in oC A 2.8 g sample of a pure metal requires 10.1 J of energy to change its temperature from 21 oC to 36 oC. What is the specific heat of the metal? s= Q = 10.1 J = 0.24 J/goC m x DT (2.8 g x 15oC) Example 2 • Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes from 32°C to 57°C.