Heat Transfer Worksheet: Temperature & Phase Changes
advertisement
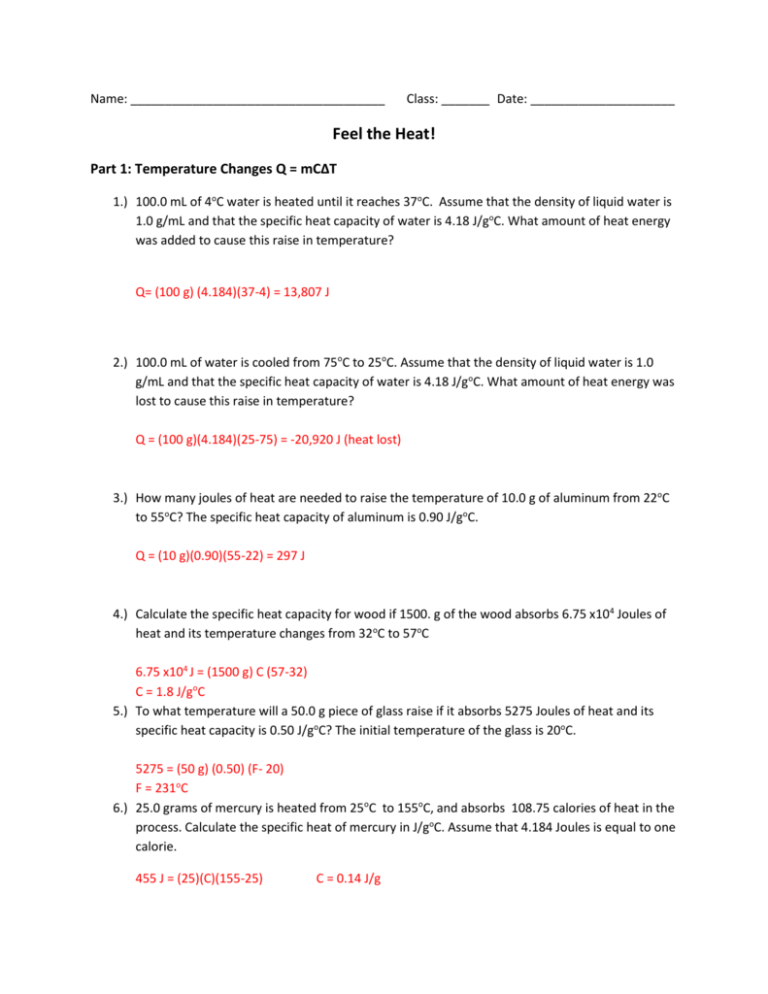
Name: _____________________________________ Class: _______ Date: _____________________ Feel the Heat! Part 1: Temperature Changes Q = mC∆T 1.) 100.0 mL of 4oC water is heated until it reaches 37oC. Assume that the density of liquid water is 1.0 g/mL and that the specific heat capacity of water is 4.18 J/goC. What amount of heat energy was added to cause this raise in temperature? Q= (100 g) (4.184)(37-4) = 13,807 J 2.) 100.0 mL of water is cooled from 75oC to 25oC. Assume that the density of liquid water is 1.0 g/mL and that the specific heat capacity of water is 4.18 J/goC. What amount of heat energy was lost to cause this raise in temperature? Q = (100 g)(4.184)(25-75) = -20,920 J (heat lost) 3.) How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22oC to 55oC? The specific heat capacity of aluminum is 0.90 J/goC. Q = (10 g)(0.90)(55-22) = 297 J 4.) Calculate the specific heat capacity for wood if 1500. g of the wood absorbs 6.75 x104 Joules of heat and its temperature changes from 32oC to 57oC 6.75 x104 J = (1500 g) C (57-32) C = 1.8 J/goC 5.) To what temperature will a 50.0 g piece of glass raise if it absorbs 5275 Joules of heat and its specific heat capacity is 0.50 J/goC? The initial temperature of the glass is 20oC. 5275 = (50 g) (0.50) (F- 20) F = 231oC 6.) 25.0 grams of mercury is heated from 25oC to 155oC, and absorbs 108.75 calories of heat in the process. Calculate the specific heat of mercury in J/goC. Assume that 4.184 Joules is equal to one calorie. 455 J = (25)(C)(155-25) C = 0.14 J/g Part 2: Phase Changes Q =mL LV = 2260 J/g LF = 334 J/g 1. What amount of energy must be added to water to 2000. g of water at 100oC to boil it? Q = (2000)(2260)= 4,520,000J 2. What mass of ice can be melted at 0oC with 12,500 J? 12500 J = m (334) m = 37.4 g 3. When 1250. J is added to 4000. g of a metal, it all melts and remains at a constant temperature, What is the heat of fusion of this metal? 1250 = (4000) (Lf) Lf = 0.3125 J/g Part 3: Putting it together. Q = mC∆T and Q = mL 1. What amount of heat is needed to boil 200.0 g of ice? The ice starts at 0oC. Q1= (200)(334) = 66,800 J Q2 = (200)(4.184)(100) = 83, 680 J Q3 = (200)(2260) = 452, 000 J Q total = 602, 480 J 2. How much heat energy must be removed from 50.0 g of water at 72oC to cool it to -10oC? Q1 = (50)(4.184)(0-72)= -15,062 J Q2 = (50)(334) = -16700 J Q3= (50)(2.01)(- 10 - 0) = -1005 J Q total = - 32, 767 J (Heat Lost)











