Heat Transfer & Calorimetry: Calculations & Heating Curves
advertisement

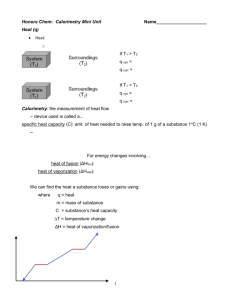
Heat (q) • Heat: the transfer of energy between objects due to a temperature difference • Flows from higher-temperature object to lower-temperature object System (T1) System (T1) Heat Heat Surroundings (T2) If T1 > T2 q system = exothermic Surroundings (T2) If T1 < T2 q system = + endothermic Calorimetry: the measurement of heat flow • device used is called a... calorimeter specific heat capacity (C): amt. of heat needed to raise temp. of 1 g of a substance 1oC (1 K) • Only useable within a state of matter (i.e. s, l, or g) For energy changes involving… heat of fusion (ΔHfus): melting/freezing heat of vaporization (ΔHvap): boiling/condensing There are NO temp changes during a phase change. Various Specific Heat Capacities Substance Specific heat capacity (J/K g) Gold 0.129 Silver 0.235 Copper 0.385 Iron 0.449 Aluminum 0.897 H2O(l) 4.184 H2O(s) 2.03 H2O(g) 1.998 Metals do not generally require much energy to heat them up (i.e. they heat up easily) Water requires much more energy to heat up We can find the heat a substance loses or gains using: q = m C DT where q = heat (J) m = mass of substance (g) (used within a given state of matter) C = specific heat (J/goC) DT = temperature change (oC) DH = heat of vap/fus (J/g) Heating Curve Temp. + s/l ΔHfus s g l Cl l/g ΔHvap – Cs HEAT Cg AND q = m ΔH (used between two states of matter or during a phase change) D = final – initial Using heat capacities… q = m C ΔT q (J) = mass (g) C (J/goC) ΔT (oC) q = joules (J) Mnemonic device: q = m “CAT” Heating Curves • Temperature Change within phase • change in KE (molecular motion) • depends on heat capacity of phase C H2O (l) = 4.184 J/goC C H2O (s) = 2.077 J/goC C H2O (g) = 2.042 J/goC (requires the most heat) (requires the least heat) • Phase Changes (s ↔ l ↔ g) • change in PE (molecular arrangement) • temperature remains constant • overcoming intermolecular forces (s ↔ l) ΔHfus = 333 J/g ΔHvap = 2256 J/g (l ↔ g) Why is this so much larger? Heating Curve of Water From Ice to Steam in Five Easy Steps q4 q5 q1: Heat the ice to 0°C q1 = m Cs ΔT q3 q2 q2: Melt the ice into a liquid at 0°C q2 = m ΔHfus q3: Heat the water from 0°C to 100°C q3 = m Cl ΔT q1 Heat q4: Boil the liquid into a gas at 100°C Heat q4 = m ΔHvap q5: Heat the gas above 100°C q5 = m Cg ΔT qtot= q1 + q2 + q3 + q4 + q5 Heating Curve Practice 1. How much energy (J) is required to heat 12.5 g of ice at –10.0 oC to water at 0.0 oC? 4 3 5 Notice that your q values are positive because heat is added… 2 1 q1: Heat the ice from -10 to 0°C q1 = 12.5 g (2.077 J/g oC)(0.0 - -10.0 oC) = 259.63 J q2: Melt the ice at 0°C to liquid at 0 oC q2 = 12.5 g (333 J/g) = 4162.5 J qtot = q1 + q2 = 259.63 J + 4,162.5 J = 4,420 J Heating Curve Practice 2. How much energy (J) is required to heat 25.0 g of ice at –25.0 oC to water at 95.0 oC? 4 3 2 1 5 Notice that your q values are positive because heat is added… q1: Heat the ice from -25 to 0°C q1 = 25.0 g (2.077 J/g oC)(0.0 - -25.0 oC) = 1298.1 J q2: Melt the ice at 0°C to liquid at 0 oC q2 = 25.0 g (333 J/g) = 8325 J q3: Heat the water from 0°C to 95 °C q3 = 25.0 g (4.184 J/g oC)(95.0 – 0.0oC) = 9937 J qtot = q1 + q2 + q3 = 1298.1 J + 8,325 J + 9937 J = 19,560 J Heating Curve Practice 3. How much energy (J) is removed to cool 50.0 g of steam at 115.0 oC to ice at -5.0 oC? 4 3 2 1 5 Notice that your q values are negative because heat is removed… q5: Cool the steam from 115.0 to 100°C q5 = 50.0 g (2.042 J/g oC)(100.0 - 115.0 oC) = -1531.5 J q4: Condense the steam into liquid at 100°C q4 = 50.0 g ( - 2256 J/g) = -112,800 J q3: Cool the water from 100°C to 0 °C q3 = 50.0 g (4.184 J/g oC)(0.0 – 100.0oC) = -20920 J q2: Freeze the water into ice at 0 °C q2 = 50.0 g (- 333 J/g) = -16650 J q1: Cool the ice from 0°C to – 5.0 °C q1 = 50.0 g (2.077 J/g oC)(- 5.0 – 0.0oC) = -519.25 J qtot = q1 + q2 + q3+ q4 + q5 = -1531.5 J + -112,800 J + -20920 J + -16,650 J + -519.25 J = -152,000 J Food and Energy Caloric Values Food joules/gram calories/gram “Calories”/gram Protein 17,000 4,000 4 Fat 38,000 9,000 9 Carbohydrates 17,000 4,000 4 1 calorie = 4.184 joules 1000 calories = 1 “Calorie” "science" "food" or… 1 Kcal = 1 “Calorie” Smoot, Smith, Price, Chemistry A Modern Course, 1990, page 51 Does water have negative calories? How many Calories (nutritional) will you burn by drinking 1.0 L of water, initially at 36.5 oF (standard refrigeration temperature)? Assume that the body must expend energy to heat the water to body temperature at 98.6 oF. 37 oC 1 L = 1000 mL 2.5 oC 5 C F 32 1 mL = 1 g 9 1 calorie = 4.184 joules q mCDT 1000 calories = 1 “Calorie” q = 1.0 x 103 g (4.184 J/g oC)(37 oC - 2.5 oC) = 144,348 J 144348 J 1 cal 1 “Cal” 4.184 J 1000 cal = 35 Cal What will happen over time? Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 291 Let’s take a closer look… Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 291 Eventually, the temperatures will equalize Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 291 Thermometer Much calorimetry is carried out using a coffee-cup calorimeter, under constant pressure (i.e. atmospheric pressure) • If we assume that no heat is lost to the surroundings, then the energy absorbed inside the calorimeter must be equal to the energy released inside the calorimeter. i.e., q absorbed = – q released qx = – qy Styrofoam cover Styrofoam cups Stirrer Heat Transfer Experiments 1. A 75.0 g piece of lead (specific heat = 0.130 J/goC), initially at 435oC, is set into 125.0 g of water, initially at 23.0oC. What is the final temperature of the mixture? Pb 75.0 g 125 g 435.0 °C 23.0 °C C = 0.130 J/°C g What is the final temperature, Tf, of the mixture? qwater = –qPb q = m x C x ΔT for both cases, although specific values differ Plug in known information for each side Solve for Tf ... A 75.0 g piece of lead (specific heat = 0.130 J/goC), initially at 435oC, is set into 125.0 g of water, initially at 23.0oC. What is the final temperature of the mixture? q = m x C x ΔT for both cases, although specific values differ Plug in known information for each side qwater = –qPb mwater Cwater DTwater = –mPb CPb DTPb 125 (4.18) (Tf – 23) = –75 (0.13) (Tf – 435) 522.5 Tf – 12017.5 = +9.75 Tf +12017.5 532.25 Tf –9.75 Tf + 4241.25 +9.75 Tf +12017.5 = Tf = 30.5oC 16258.75 2. A 97.0 g sample of gold at 785oC is dropped into 323 g of water, which has an initial temperature of 15.0oC. If gold has a specific heat of 0.129 J/goC, what is the final temperature of the mixture? Assume that the gold experiences no change in state of matter. T = 785oC Au mass = 97.0 g T = 15.0 oC mass = 323 g - LOSE heat = GAIN heat - [(C Au) (mass) (DT)] = (C H2O) (mass) (DT) - [(0.129 J/goC) (97 g) (Tf - 785oC)] = (4.184 J/goC) (323 g) (Tf - 15oC)] - [(12.5) (Tf - 785oC)] = (1.35 x 103) (Tf - 15oC)] -12.5 Tf + 9.82 x 103 = 1.35 x 103 Tf - 2.02 x 104 3 x 104 = 1.36 x 103 Tf Tf = 22.1oC HW #2. If 59.0 g of water at 13.0 oC are mixed with 87.0 g of water at 72.0 oC, find the final temperature of the system. T = 72.0 oC mass = 87.0 g T = 13.0 oC mass = 59.0 g - LOSE heat = GAIN heat - [ (mass) (C H2O) (DT)] = (mass) (C H2O) (DT) - [ (59 g) (4.184 J/goC) (Tf - 13oC)] = (87 g) (4.184 J/goC) (Tf - 72oC)] - [(246.8) (Tf - 13oC)] = (364.0) (Tf - 72oC)] -246.8 Tf + 3208 = 364 Tf - 26208 29416 = 610.8 Tf Tf = 48.2oC HW #4. 240. g of water (initially at 20.0oC) are mixed with an unknown mass of iron initially at 500.0oC (CFe = 0.4495 J/goC). When thermal equilibrium is reached, the mixture has a temperature of 42.0oC. Find the mass of the iron. Fe T = 500oC mass = ? grams T = 20oC mass = 240 g - LOSE heat = GAIN heat -q1 = q2 - [ (mass) (CFe ) (DT)] = (mass) (CH2O) (DT) - [ (X g) (0.4495 J/goC) (42oC - 500oC)] = (240 g) (4.184 J/goC) (42oC - 20oC)] - [ (X) (0.4495) (-458)] = (240 g) (4.184) (22) 205.9 X = 22091 X = 107 g Fe A 23.6 g ice cube at –31.0oC is dropped into 98.2 g of water at 84.7oC. Find the equilibrium temperature. KEY: Assume that the ice melts and the final product is a liquid. qice = –qwater qwater = –98.2 (4.18) (Tf – 84.7) = –410.48 Tf + 34767.32 qice = 23.6 (2.077) (0 – –31) + 23.6 (333) + 23.6 (4.18) (Tf – 0) = 1519.53 = 9378.33 + 98.65 Tf + 7858.8 + 509.13 Tf = 25388.99 Tf = 49.9oC 98.65 Tf Heating Curve Challenge Problems 1. A sample of ice at is placed into 75 g of water initally at 85oC. If the final temperature of the mixture is 15oC, what was the mass of the ice? 52.8 g ice Temperature (oC) -25oC 140 120 100 80 60 40 20 0 -20 -40 -60 -80 -100 DH = mol x DHvap DH = mol x DHfus Heat = mass x Dt x Cp, gas Heat = mass x Dt x Cp, liquid Heat = mass x Dt x Cp, solid Time 2. A 38 g sample of ice at -5oC is placed into 250 g of water at 65oC. Find the final temperature of the mixture assuming that the ice sample completely melts. 45.6 oC 3. A 35 g sample of steam at 116oC are bubbled into 300 g water at 10oC. Find the final temperature of the system, assuming that the steam condenses into liquid water. 76.6 oC Heating Curve for Water (Phase Diagram) q4 = m DHvap DHvap = +/- 2256 J/g 140 120 Temperature (oC) 100 q2 = m DHfus DHfus = +/- 333 J/g BP 80 60 0 B 2 -20 -40 q5 = m C D T C g = 2.042 J/goC C AB BC CD DE ED EF 1 q1 = m C D T Cs = 2.077 J/goC -60 -80 4 q3 = m C D T Cl = 4.184 J/goC 20 MP 5 E D 3 40 F A -100 Heat warm ice melt ice (s l) warm water boil water (l g) condense steam (g l) superheat steam Calculating Energy Changes Heating Curve for Water 140 120 DH = mol x DHfus DH = mol x DHvap Temperature (oC) 100 80 Heat = mass x Dt x Cp, gas 60 40 20 0 Heat = mass x Dt x Cp, liquid -20 -40 -60 -80 Heat = mass x Dt x Cp, solid -100 Time