Molar Volume, Density and Molar Mass
advertisement
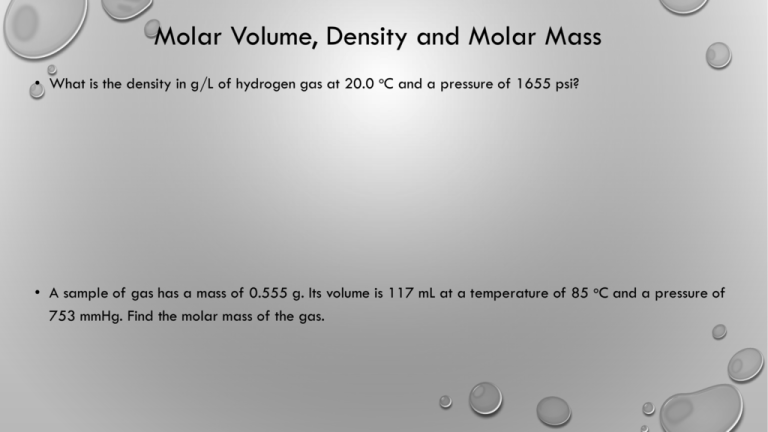
Molar Volume, Density and Molar Mass • What is the density in g/L of hydrogen gas at 20.0 oC and a pressure of 1655 psi? • A sample of gas has a mass of 0.555 g. Its volume is 117 mL at a temperature of 85 oC and a pressure of 753 mmHg. Find the molar mass of the gas. Dalton’s Law of Partial Pressures. • A gas mixture with a total pressure of 745 mmHg contains each of these three gases at the indicated partial pressures. The mixture also contains helium gas. What is the partial pressure of the helium gas? What mass of helium gas is present in a 12.0 L sample of this mixture at 273K • CO2, 125 mmHg, • Ar, 214 mm Hg • O2, 187 mmHg • He, ? Dalton’s Law • An apparatus consists of a 4.0L flask containing nitrogen gas at 25 C and 803 kPa joined by a valve to a 10.0L flask containing argon gas at 25 C and 47.2 kPa. The valve is opened and the gases mix. (a) what is the partial pressure of each gas after mixing? B) what is the total pressure of the gas mixture. Hint 1: Dalton’s law says that you can treat each gas individually. Hint 2: Treat as two different problems, one for each gas. 10.0L 25 C 47 kPa 4.0L 25 C 803 kPa Hint 3: Then add to get total pressure. What is the partial pressure of Nitrogen? A) 321kPa B) 850 kpa C) 47 kPa D) 229 kPa E) none of the above For the given reaction, if 2.2 moles of C3H8 is reacted with 8.0 moles of O2, how many moles of CO2 are produced? If all propane is reacted: If all O2 is reacted: What is the pressure of the final sample if it is contained within a 1.00L container at 30.0oC 𝑛𝑅𝑇 𝑃= = 𝑉