Gas Laws Worksheet: Gay-Lussac & Combined Gas Law
advertisement

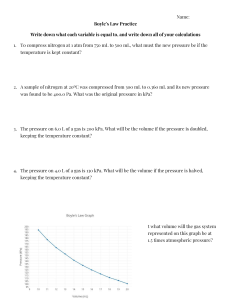
Honors Chemistry Gas Laws Worksheet #2 Name ______________________________ Hour ____ Gay-Lussac’s Law 1. Equation for Gay-Lussac’s Law: 2. How are the two variables related to each other? 3. Why do we use Kelvin when doing calculations that involve gases? 4. A gas in a closed container has a pressure of 300 kPa at 30.2 °C. What will the pressure be if the temperature drops to -172.8 °C? 5. A gas has a pressure of 6.58 kPa at 539 K. What will the pressure be at 211 K if the volume does not change? 6. The pressure in a car tire is 1.84 atm at 27.5 °C. At the end of a trip on a hot, sunny day, the pressure has risen to 2.49 atm. What is the temperature of the air in the tire? 7. The gas left in a used aerosol can is at a pressure of 103.4 kPa at 25.6 °C. If this can is thrown onto a campfire, what is the pressure of the gas when its temperature reaches 928.1 °C? Combined Gas Law 8. Equation for the Combined Gas Law: 9. How do you determine which variable had a bigger impact on your final answer? 10. The volume of a gas decreased from 2 L to 1 L. The temperature of a gas increased from 20 °C to 100 °C. If the initial pressure is 752.4 torr, what is the new pressure? 11. The volume of a gas increases from 752.7 mL to 1505.4 mL. The pressure of the gas decreased from 3 atm to 2 atm. If the initial temperature was 25 °C, what is the final temperature (in °C)? 12. You have 56.7 mL of a gas. Its temperature changes from 290.1 K to 303.7 K. Its pressure changes from 682.7 mmHg to 700.3 mmHg. What is the new volume? 13. In problem #12, which variable had more impact on the final answer? Why? 14. You have 25 cm3 of a gas at 25 °C and 103.86 kPa. What is the new volume at STP? 15. In problem #14, which variable had more impact on the final answer? Why?