12.2 Ideal Gasses and the Ideal Gas Law
advertisement
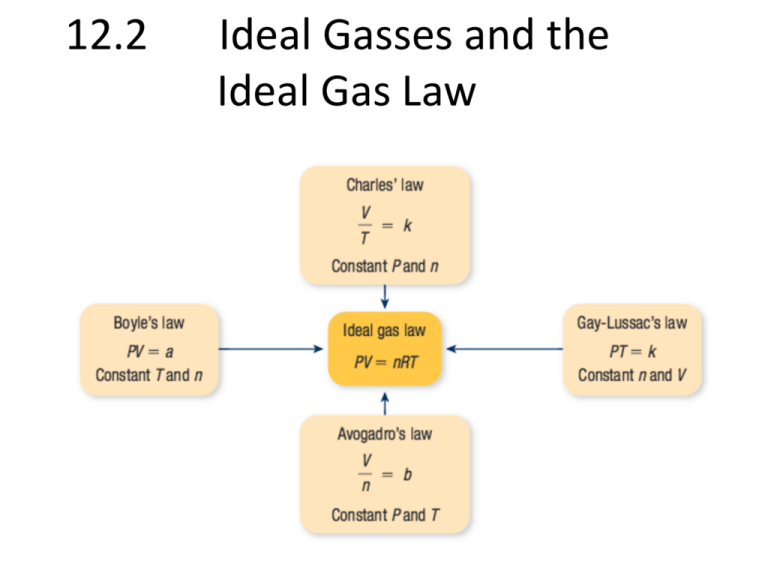
12.2 Ideal Gasses and the Ideal Gas Law An Ideal Gas • All gas laws are based on the assumption that gasses behave “ideally” • An ideal gas: – Moves in all directions and in straight lines – There is no loss in kinetic energy in collisions – Particles have no size and are always the same volume as the container – There are no intermolecular forces – Does not condense into a liquid when cooled An Ideal Gas • Ideal gasses do not exist • “Ideal” is a convenient approximation that works well to predict the behaviour of gases. • At ordinary conditions, most gases obey the gas laws and their behaviour resembles that of an ideal gas The Ideal Gas Law • By combining gas laws we can come up with the ideal gas law: – Charles’ Law: V/T = k – Boyle’s Law: PV = a – Avogadro’s Law: V/n = b • Combining the laws gives you: – PV/nT = R Where R is a constant incorporating k, a and b The Ideal Gas Law PV = nRT Where: P is the pressure in kPa V is the volume in L n is the amount in moles T is the temperature in K R is the universal gas constant: 8.314 kPa.L/mol.K Example 1. The Goodyear blimp has a volume of 2.5 x107L and usually operates with the gas at a temperature of 12 °C and a pressure of 112 kPa. What amount of helium does the blimp contain? What is the mass of this amount of helium? Example 2. Calculate the volume of 32.4g of nitrogen gas, N2(g), in a container at 25°C and 96.4kPa. 2. Determine the density of 1.00mol of pure carbon dioxide gas at STP (0°C, 101.3kPa). 3. 10.24 g of a pure gas occupies 2.10L at 123°C and 99.7 kPa. Calculate the molar mass of this gas. Homework Read Section 12.2 Questions p.587 # 1-4 Extra Practice p. 589 # 4-10 (optional)