Ideal Gas Law
advertisement

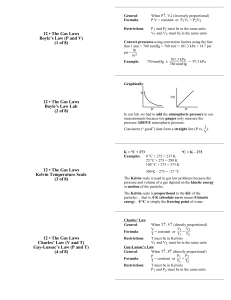
Ideal Gas Law What is the Ideal Gas Law? An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly elastic and in which there are no intermolecular attractive forces. In such a gas, all the internal energy is in the form of kinetic energy and any change in internal energy is accompanied by a change in temperature. Measurements necessary to accurately describe a sample of gas: An ideal gas can be characterized by four variables: • pressure • volume • temperature • number of moles Mathematical Expression PV = nRT P = pressure V = volume T = temperature n = number of moles R = gas constant (R = 0.0821 if P = atm, V = L, and T = K) (R = 8.314 if P = Pa, V = m3, and T = K) Practice Problems 1.What volume would be occupied by 100g of oxygen gas (O2) at a pressure of 1.5 atm and a temperature of 25°C? P= V= n= R= T= 2. A balloon is inflated with 0.2495g of helium (He) to a pressure of 1.26 atm. If the desired volume of the balloon is 1.25L, what must the temperature be? P= V= n= R= T= 3. A welder’s acetylene tank has a volume of 75m3. It is stored at a temperature of 23.24°C and a pressure of 76,670Pa. How many moles of acetylene are in the tank? P= V= n= R= T= 4. A sample of bromine gas (Br2) is loaded into an evacuated demonstration bottle at STP (1atm and 273K). The volume of the bottle is 0.25L. How many moles of Br2 will be contained in the bottle? P= V= n= R= T= Dalton’s Law of Partial Pressures The sum of the partial pressures of all the components in a gas mixture is equal to the total pressure of the gas mixture. PT = Pa + Pb + Pc …… Practice Problems 1. What is the pressure of a mixture of helium, nitrogen, and oxygen if their partial pressures are 600, 150, and 102 mmHg respectively? 2. Flask contains a mixture of hydrogen gas and oxygen gas. The pressure being exerted by these gases is 785 mmHg, as determined by a manometer. If the partial pressure of hydrogen gas in the mixture is 395 mmHg, what is the partial pressure of the oxygen gas? 3. What is the atmospheric pressure of the partial pressures of nitrogen, oxygen, and argon are 77.75 kPa, 9.94 kPa, and 1.99 kPa, respectively?