Gas Laws Worksheet: Chemistry Problems & Conversions
advertisement
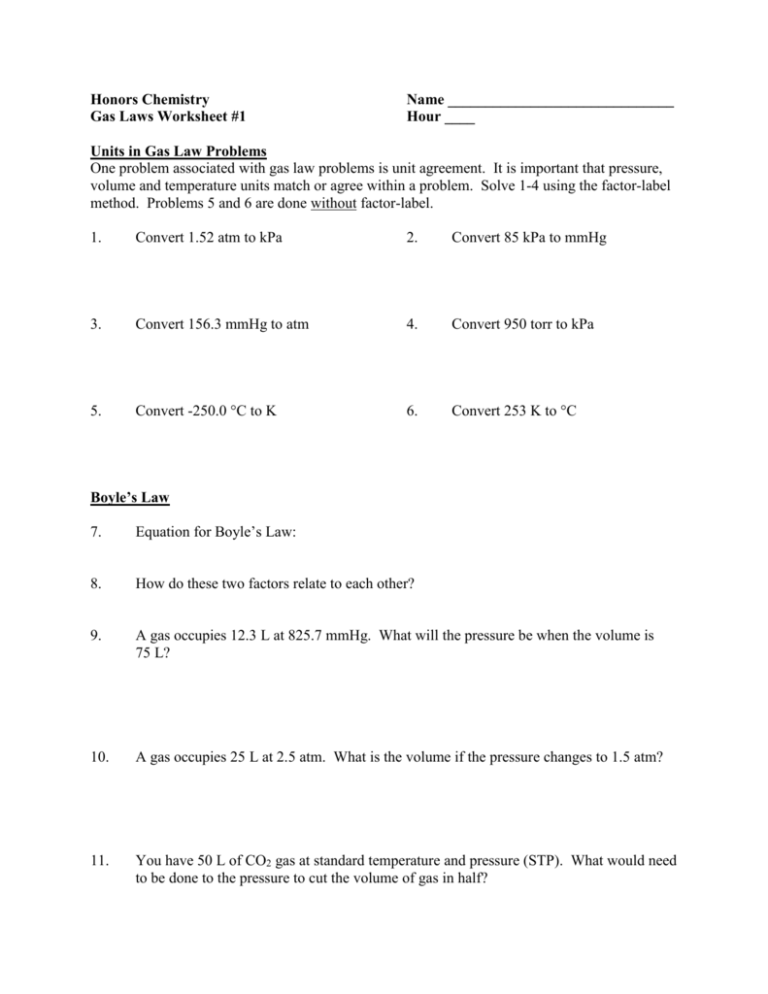
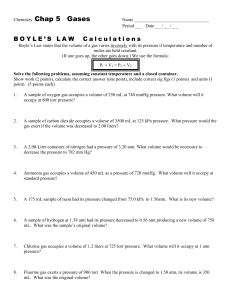
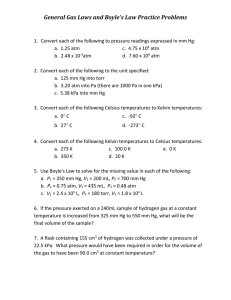
Honors Chemistry Gas Laws Worksheet #1 Name ______________________________ Hour ____ Units in Gas Law Problems One problem associated with gas law problems is unit agreement. It is important that pressure, volume and temperature units match or agree within a problem. Solve 1-4 using the factor-label method. Problems 5 and 6 are done without factor-label. 1. Convert 1.52 atm to kPa 2. Convert 85 kPa to mmHg 3. Convert 156.3 mmHg to atm 4. Convert 950 torr to kPa 5. Convert -250.0 °C to K 6. Convert 253 K to °C Boyle’s Law 7. Equation for Boyle’s Law: 8. How do these two factors relate to each other? 9. A gas occupies 12.3 L at 825.7 mmHg. What will the pressure be when the volume is 75 L? 10. A gas occupies 25 L at 2.5 atm. What is the volume if the pressure changes to 1.5 atm? 11. You have 50 L of CO2 gas at standard temperature and pressure (STP). What would need to be done to the pressure to cut the volume of gas in half? Charles’ Law 12. Equation for Charles’ Law: 13. How do these two factors relate to each other? 14. What does temperature actually measure? 15. At 27.8 °C, a gas occupies 1500 mL. What volume will it have at 100.0 °C? 16. What temperature (in K) must a gas be if it occupied 1.396 L at 72.3 °C and now occupies 1.044 L? 17. At -32.7 °C, a gas takes up 0.750 mL. What temperature, in °C, would be needed to reduce the volume to half that amount? 18. A volume of 2.15 mL of Oxygen was collected in the lab at a temperature of 17 °C and 740.2 mmHg. After a day, the volume of the gas has become 2.21 mL and the barometer reading (for air pressure) had not changed. What is the new temperature in the lab (in °C)? 19. If the temperature at which a gas is collected is doubled, what happens to the volume if pressure remains constant?