Experiment 11 IN/POST LAB assignment
advertisement

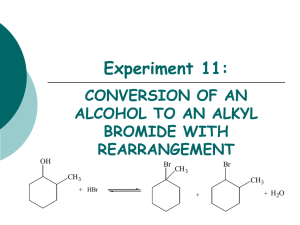
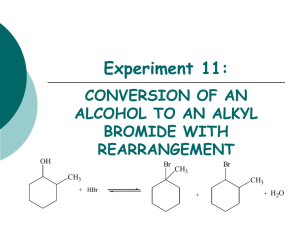
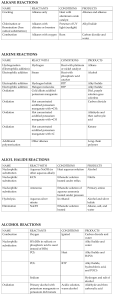
CHML211 Exp. 11 Conversion of an Alkyl Halide to an Alcohol with Rearrangement IN-LAB ASSIGNMENT: (EACH STUDENT will record the following data in his/her laboratory notebook in a well organized manner during lab period. Yellow carbon copies will be submitted for grading along with Post-Lab assignment.) …..max score = 20 pts. 6. Data/Calculations a. b. c. d. e. f. g. Initial mass of 2-methylcyclohexanol used Initial 50 mL beaker mass Final 50 mL beaker + product mass Final product mass Physical state and color of product Theoretical yield calculation (not just the value!) Percent yield calculation (not just the number!) 7. In-lab Questions (The following questions should be answered in laboratory notebook.) a. There are two possible products from the reaction of 3-methyl-2-pentanol with aq. HBr. Draw the products and a complete mechanism for their formation, including all intermediates. Name both products based on the IUPAC system and circle the major product. OH H Br ? CH3 MW: 102.18 g/mol b. Based on the reaction above, calculate the theoretical yield for the reaction based on 4.0 g of the alcohol and 20 mL of the 48% HBr. Show calculations based on both reactants! The molecular weight of the alcohol is given, however the molecular weight of the product must be determined based on the structure. Be sure to give units. CHML211 Exp. 11 Conversion of an Alkyl Halide to an Alcohol with Rearrangement POST-LAB ASSIGNMENT: (EACH LAB GROUP will submit one copy of a typewritten, paragraph style report addressing all of the points listed below. Must be written using PAST TENSE, PASSIVE VOICE. ) …..max score = 50 pts. 8. Experimental (Write 1-2 paragraphs including all of the following. Do NOT present a bulleted outline.) What type of reaction was performed? Describe the actual synthetic procedure. Include names of any reactants used and desired product, as well as name of solvent and catalyst used (if any). Be sure to give actual volumes/masses of compounds used during the synthesis (not just what the lab manual tells you to use). Describe the purification technique used to isolate the desired product. Be sure to give names and actual volumes/masses of any compounds used during the purification process. Describe the analytical technique used to evaluate the product. Be sure to give names and actual volumes/masses of any compounds used during the analytical process and how the product was identified during this process. Include any spectral analysis used. 9. Results (Complete tables. Once completed, copy/ paste completed tables into your document.) Table 11.1 Experimental results Final Mass of product (g) Theoretical Yield (g) Percent Yield Product Appearance Table 11.2 Silver nitrate test results Test Solution Reaction Rate (min/sec) 1-bromobutane 2-bromobutane 2-bromo-2-methylpropane Product sample Functional Group O-H stretch C-O stretch sp3 CH stretch C-Br stretch Table 11.3 IR Spectral analysis results 2-methylcyclohexanol Base Values (cm-1) Frequency (cm-1) 3200–3600 1000–1200 2850–3000 500–700 Alkyl halide product Frequency (cm-1) 10. Discussion (Write 1-2 pages addressing all of the following points.) Restate the name of the reactants combined and the name of the product formed during the synthesis. State the final product mass and the calculated percent yield for the synthesis. Be sure to report these values using the correct units. Based on the silver nitrate test results, what was the degree of substitution of your product? Briefly support this conclusion using the reaction rates of the provided standard solutions and product solution with silver nitrate. How can IR spectroscopy be used to differentiate between the reactant alcohol and the MAJOR product alkyl halide in this experiment? Give the identity of one type of absorption and how it could be used to determine whether or not the conversion took place. Include the typical frequency and the actual frequency for this type of bond in your statement. Be sure to include units for frequencies. Include a short comment addressing what could be done differently to improve the experimental results if repeated.