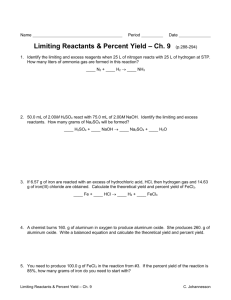
8. Percentage yield
advertisement
Percentage yield Learning objective Perform calculations to determine the percentage yield of a reaction • Key words: • Percentage yield • Limiting reagent When writing a fully balanced chemical equation, it is assumed that ALL of the reactants will be converted into products. If this were true then the yield would be 100% The percentage yield can be calculated using the formula: 𝑎𝑐𝑡𝑢𝑎𝑙 𝑎𝑚𝑜𝑢𝑛𝑡, 𝑖𝑛 𝑚𝑜𝑙, 𝑜𝑓 𝑝𝑟𝑜𝑑𝑢𝑐𝑡 % 𝑦𝑖𝑒𝑙𝑑 = 𝑥 100 𝑡ℎ𝑒𝑜𝑟𝑒𝑡𝑖𝑐𝑎𝑙 𝑎𝑚𝑜𝑢𝑛𝑡, 𝑖𝑛 𝑚𝑜𝑙, 𝑜𝑓 𝑝𝑟𝑜𝑑𝑢𝑐𝑡 Factors that affect the percentage yield: • The reaction may be at equilibrium and may not go to completion • Other side reactions may occur, leading to byproducts • The reactants may not be pure • Some of the reactants or products may be left behind in the apparatus used in the experiment • Separation and purification may result in the loss of some of the product 1. A student adds 200.0g of C7H6O3 to an excess of C4H6O3, this produces C9H8O4 and C2H4O2. Calculate the percent yield if 231 g of aspirin (C9H8O4) is produced. C7H6O3 + C4H6O3 C9H8O4 + C2H4O2 2. According to the following equation, Calculate the percentage yield if 550.0 g of toluene ()added to an excess of nitric acid () provides 305 g of the p-nitrotoluene product. C7H8 + HNO3 C7H7NO2 + H2O 3. Aluminum reacts with an aqueous solution containing excess copper (II) sulfate. If 1.85 g Al reacts and the percentage yield of Cu is 56.6%, what mol mass of Cu is produced? Al + CuSO4 Cu + Al2(SO4)3 4. The combustion of methane produces carbon dioxide and water. Assume that 2.0 mol of CH4 burned in the presence of excess air. What is the percentage yield if the reaction produces 87.0 g of CO2? Answers: 1. 88.34% 2. 37.20% 3. 2.46g 4. 98.50%