CHM 253L Organic Chemistry Laboratory I
advertisement

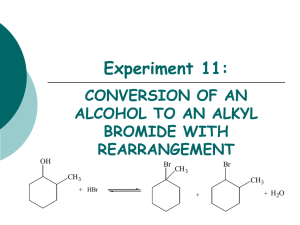
CHM 253L Organic Chemistry Laboratory I Laboratory Follow-Up Assignment #7 Due: Tuesday, December 4, 2007 by 5:00 pm Part 1 You should have two NMRs that were handed out for the “competitive nucleophiles” part of the experiment (you only received the data, but did not actually perform this experiment). You should use this information and prepare a RESULTS and DISCUSSION section. Be careful here as I want you to write it up as if you were publishing in a peer-reviewed journal (like Journal of Organic Chemistry or Organic Letters). The following are some guiding questions that will help you construct a good R/D narrative. 1. Identify the important peaks in each 1H-NMR spectrum and their integral values that will be needed to determine the relative percentage of each alkyl halide in your mixture. 2. Show the calculations for the percentages of each alkyl halide in both mixtures. 3. Identify the stronger nucleophile in both reactions and provide a brief explanation as to how you came to this conclusion. You may want to consult sections 6.5 and 7.4 of the Jones text. 4. For which reaction is the difference in nucleophilic strength more important? Explain, in detail, why your results do or do not make sense according to what you know about the SN2 and SN1 reactions. Part 2 The second part of this experiment involves an investigation of reaction kinetics especially as it pertains to the effect of solvent. The following information should be turned in for this part of the experiment: 1. Write a balanced SN1 mechanism between the halide and the nucleophile(s) in the solution you studied. HINT: be careful -- you need to really think about what are the major species in solution and their relative concentrations!!!!! 2. Write a balanced SN2 mechanism between the halide and the nucleophile(s) in the solution you studied. HINT: be careful -- you need to really think about what are the major species in solution and their relative concentrations!!!!! 3. Write a balanced E1 mechanism between the halide and the base(s) in the solution you studied. HINT: be careful -- you need to really think about what are the major species in solution and their relative concentrations!!!!! 4. Write a balanced E2 mechanism between the halide and the base(s) in the solution you studied. HINT: be careful -- you need to really think about what are the major species in solution and their relative concentrations!!!!! 5. Use the compiled class data (available in the Organic Chemistry folder on Andromeda) to create a series of bar graphs (EXCEL) to show any trends you have discovered between a. different alkyl halides in same solvent b. same alkyl halides in different solvents 6. Compare/contrast the graphs (What do the graphs indicate or suggest?) that you generated in question #5. In particular, you should consider the following situations: a. Using a different set of alkyl halides (e.g. 2-chloro-2-methylpropane and 2bromo-2-methyl-propane) in the same solvent system. b. Using the same alkyl halide (e.g. either 2-chloro-2-methylpropane or 2-chloro-2methylbutane) in the two different solvent systems. 7. Using sound chemical theory/logic, describe the reasons for any differences among the data sets in question #6. Individual data (choose ONE set of experiments that you performed): 8. Was there a chemical reaction in Flask #1? How do you know? If there was a chemical reaction in Flask #1 describe the reaction by means of a balanced chemical equation. 9. Was there a chemical reaction in Flasks #2-#5? How do you know? 10. If there were chemical reactions in Flasks #2-#5, what observations did you make about the similarities and differences in each reaction? Using sound chemical theory/logic, describe the reasons for the differences and similarities between the reactions in Flasks #2-#5. I am asking you here to think like the molecules in the flask. What types of intermolecular forces are occurring in each case? Again, I think it would be helpful to consult Jones sections 6.5 and 7.4. 11. What mechanism (from the menu of possibilities from questions #1-4) best explains the type of reaction occurring in flasks #2-5? Why? 12. Is an additional experiment needed to verify the mechanism? If so, describe it and what information the experiment would provide. 13. How might the results of the experiment been different if the substrate was 2-fluoro-2methylpropane? 14. How might the results of the experiment been different if the solvent system was changed to methanol-water? Conclusions: 15. In conclusion, what statement can you make about the role of solvent in this experiment? 16. What statement can you make about the role of the halogen in this experiment? Enrichment Exercise Complete the SPARTAN molecular modeling exercise that is available via the assessment page on the organic chemistry web site. You’ll need to decide which algorithm to employ to obtain energy data and to acquire the electrostatic potential map.