Ch. 6: Thermochemistry
advertisement

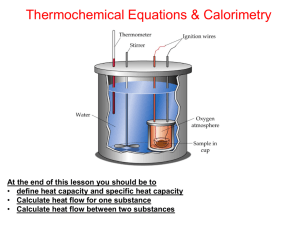
Thermochemistry Section 2: Enthalpy and Calorimetry Enthalpy • definition: H = E + PV • since E, P and V are all state functions, then H is too • for the following, the process is at constant P and the only type of work allowed is PV work ∆E = qP + w = qP - P∆V H = E + PV qP = ∆ E +P∆V ∆H = ∆E+P∆V so, qP = ∆H at constant P Enthalpy • heat of reaction and change in enthalpy are used interchangeably for a reaction at constant P ∆H = Hproducts - Hreactants endo: + ∆H exo: - ∆H Calorimetry • science of measuring heat • calorimeter- device used to experimentally find the heat associated with a chemical reaction • substances respond differently when heated Heat Capacity • (C) how much heat it takes to raise a substance’s T by one °C or K • the amount of energy depends on the amount of substance heat absorbed (in J) heat capacity C increase in T (in C or K) Heat Capacity • specific heat capacity – (s) heat capacity per gram – in J/C°*g or J/K*g • molar heat capacity – heat capacity per mole – in J/C°*mol or J/K*mol Constant-Pressure Calorimetry • uses simplest calorimeter (coffee-cup calorimeter) since it is open to the air • used to find changes in enthalpy (heats of reaction) for reactions occurring in a solution since qP = ∆H • heat of reaction is an extensive property, so we usually write them per mole so they are easier to use Constant-Pressure Calorimetry • when 2 reactants are mixed and T increases, the chemical reaction must be releasing heat so it is exothermic • the released energy from the reaction increases the motion of molecules, which in turn increases the T Constant-Pressure Calorimetry • If we assume that the calorimeter did not leak energy or absorb any itself (that all the energy was used to increase the T), we can find the energy released by the equation: E released by rxn = E absorbed by soln ∆H = qP = sP x m x ∆T Constant-Volume Calorimetry • uses a bomb calorimeter • weighed reactants are placed inside the rigid, steel container and ignited • water surrounds the reactant container so the T of it and other parts are measured before and after reaction Constant-Volume Calorimetry • Here, the ∆V = 0 so -P∆V = w = 0 ∆E = q + w = qV for constant volume E released by rxn = ∆T x Ccalorimeter Example 1 • When 1 mol of CH4 is burned at constant P, 890 kJ of heat is released. Find ∆H for burning of 5.8 g of CH4 at constant P. • 890 kJ is released per mole of CH4 1mol eCH 4 890kJ 5.8 gCH 4 320kJ 16.04276 gCH 4 1mol CH 4 Example 2 • When 1.00 L of 1.00 M Ba(NO3)2 solution at 25.0°C is mixed with 1.00 L of 1.00 M Na2SO4 solution at 25.0°C in a coffee-cup calorimeter, solid BaSO4 forms and the T increases to 28.1°C. The specific heat capacity of the solution is 4.18 J/g*°C and the density is 1.0 g/mL. Find the enthalpy change per mole of BaSO4 formed. Example 2 • Write the net ionic equation for the reaction: Ba2+ (aq) + SO42- (aq) BaSO4(s) • Is the energy released or absorbed? What does that mean about ∆H and q? exothermic: -∆H and –qP • How can we calculate ∆H or heat? heat = q = sP x m x ∆T • How can we find the m? use density and volume Example 2 • Find the mass: m D m D V V 3 3 (1.0 g / mL)(2.0 10 mL) 2.0 10 g • Find the change in T: T 28.1 25.0 3.1 C • Calculate the heat created: J 3 (4.18 )(2.0 10 g )(3.1 C ) 2.6 10 Cg Example 2 • since it is a one-to-one ratio and the moles of reactants are the same, there is no limiting reactant • 1.0 mol of solid BaSO4 is made so ∆H= -2.6x104 J/mol = -26 kJ/mol Example 3 • Compare the energy released in the combustion of H2 and CH4 carried out in a bomb calorimeter with a heat capacity of 11.3 kJ/°C. The combustion of 1.50 g of methane produced a T change of 7.3°C while the combustion of 1.15 g of hydrogen produced a T change of 14.3°C. Find the energy of combustion per gram for each. Example 3 • methane: CH4 H Ccalorimeter T kJ H (11.3 ) (7.3C ) 83kJ C H 83kJ / 1.5 g 55kJ / g • hydrogen: H2 H Ccalorimeter T kJ H (11.3 ) (14.3C ) 162kJ C H 162kJ / 1.15 g 141kJ / g • The energy released by H2 is about 2.5 times the energy released by CH4