2.0 Thermochemical Eqns & Calorimetry
advertisement

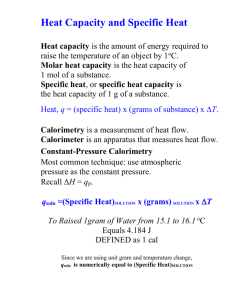
Thermochemical Equations & Calorimetry At the end of this lesson you should be to • define heat capacity and specific heat capacity • Calculate heat flow for one substance • Calculate heat flow between two substances Heat capacity • This can be calculated using the equation: • Q (q) = C ΔT where C is the heat capacity of the system. • ΔT = change in temperature (T2-T1) • Q = heat • The heat capacity is the amount of heat required to raise the temperature of the system by 10C or 1 K. • Units are J/ 0C or J/K At the end of this lesson you should be to • define heat capacity Specific heat capacities – don’t write Aluminum Copper Gold Iron Sodium Mercury Ice Water Steam Methanol Ethanol 0.900 0.387 0.128 0.444 1.23 0.138 2.01 4.18 2.01 2.918 2.454 Specific heats in J/goC at 101.3 kPa and 25oC (except for ice and steam). At the end of this lesson you should be to • define heat capacity and specific heat capacity Calculations Involving Heat Flow • Heat with only one substance • How much heat, in kJ, is required to raise the temperature of 120 g Al from 250C to 1000C ? • • • • • • • • (Specific Heat of Al is 0.902 J /g 0C) Ans. 8118 J Q=? At the end of this lesson you should be to m = 120 g • define heat capacity and 0 T1= 25 C specific heat capacity ∆T= 100 - 25 =75 • Calculate heat flow for one T2= 100 0C substance C = 0.902 J /g 0C Q = mc∆T = 120g x 0.902 J /g 0C x 75 0C = 8118 J = 8.1 kJ Worksheet - SPECIFIC HEAT CAPACITY AND HEAT TRANSFER Calculations Involving Heat Flow • Heat involved with two substances • A piece of iron (Fe) weighing 40 g is heated to 800C and dropped into 100 g of water at 250C. (Specific heat of Fe is 0.446 J /g0C and Specific heat of H2O is 4.182 J /g0C) What is the temperature when thermal equilibrium has been reached ? (Ans = 27.50C) • Heat lost by iron is equal to heat gained by the water. • -Q iron = Q water • -(miron x Ciron x ∆Tiron) = (mwater x Cwater x ∆Twater) • Basic math skills, negative sign is important. • Finish the question above then go to Worksheet – CALORIMETRY section At the end of this lesson you should be to • define heat capacity and specific heat capacity • Calculate heat flow for one substance • If Specific heat capacity the system is a pure substance with a mass of “ m ” grams • Q = m c ΔT where c is the specific heat of the substance. • The specific heat is the amount of heat required to raise one gram of the substance by 1oC or 1 K. • Units are J/ g0C or J/ g K) At the end of this lesson you should be to • define heat capacity and specific heat capacity Measuring heat transfer in a lab • A calorimeter measures the amount of heat transferred during a reaction. • Coffee Cup Calorimeter is open to the atmosphere it is called a constant pressure calorimeter • The heat capacity of the coffee cup calorimeter is assumed to be zero At the end of this lesson you should be to • define calorimeter and calorimetry Enthalpy change for a reaction • There are five ways to determine or calculate enthalpy change for a reaction; • • • • • Experiment / calorimetry Hess’ Law Use data of standard enthalpy of formation (ΔHf) Use data of standard enthalpy of combustion (ΔHc) Use data of standard enthalpy of atomization (ΔHa) or Energy bonding (D). At the end of this lesson you should be to • define calorimeter and calorimetry • Know five (5) ways to determine enthalpy change for a reaction Using calorimeter to determine enthalpy of reaction • During an experiment the solution absorbs or releases heat • Qsol = mC∆T • m = mass of solution • C = specific heat capacity of water • Qreaction = - Qsolution • If reaction/system releases heat, so surroundings (solution/water and calorimeter) absorb heat. • Temperature increase Solution absorbs heat therefore the reaction is exothermic • Temperature decrease solution releases heat therefore the reaction is endothermic. • (ΔHrxn = Qrxn/n) • Assumptions - For dilute acid or bases the density is close to water therefore you can measure the volume and calculate the mass and use the C for water in all the calculations. At the end of this lesson you should be to • define calorimeter and calorimetry • Know five (5) ways to determine enthalpy change for a reaction • Do calculations for enthaphy of reaction using calorimetry