Calorimetry

Definition: Calorimetry is the accurate and precise measurement of heat flow for chemical and physical processes.
It operates under the law of conservation of energy (what is that?)
Heat loss = Heat gained
A device used to measure the absorption or release of this heat is called Calorimeter
Watch a video lecture for an example.
o o
Open to page 511
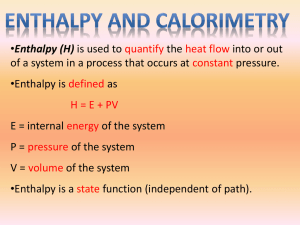
Constant-Pressure Calorimeter
(simple calorimeter) o Because it is open to a constant atmospheric pressure o Heat content of a system at constant pressure is know as Enthalpy (H) of the system o
Change in Enthalpy (ΔH) measures the amount of heat release or absorbed by a reaction at constant pressure.
o q= ΔH
Minimizing Heat loss in a simple calorimeter
Convection
Radiation
Conduction
MEASURING ENTHALPY
CHANGE
q= CmΔT q
q sys =
-q sys = sys = q surr surr
ΔH = -q q surr =
ΔH = - m. C. ΔT
- m. C. ΔT
ΔH = negative = exothermic
ΔH = positive = endothermic
Open to page 512
Constant-Volume Calorimeter
(Bomb calorimeter)
Bomb calorimeter is an insulated device containing a sealed vessel used to measure the heat released during a combustion reaction.
It is the most reliable device for measuring specific heat of a substance
It is performed at constant volume of oxygen at high pressure.
•
A chemical equation that includes the enthalpy change (ΔH)