Beer`s Law
advertisement
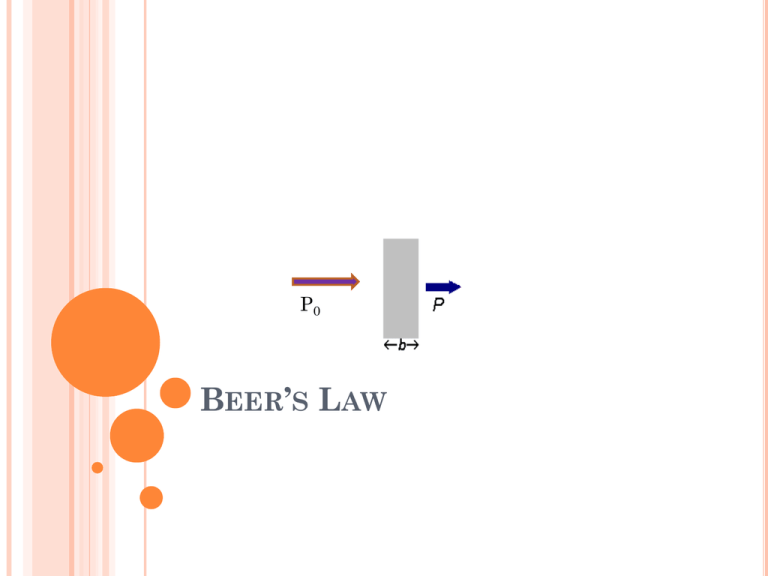
P0 BEER’S LAW USES OF BEER’S LAW - Relates concentration to the optical measurement of ‘absorbance’ - combined with spectrophotometry can be used to distinguish and compare different molecules in solution THE RELATIONSHIP BETWEEN ABSORBANCE AND TRANSMITTANCE IS ILLUSTRATED IN THE FOLLOWING DIAGRAM: Transmittance 0% 100% 2.0 0 Absorbance THE AMOUNT OF RADIATION ABSORBED MAY BE MEASURED IN A NUMBER OF WAYS: Transmittance, T = P / P0 % Transmittance, %T = 100 T Absorbance, A = log10 P0 / P A = log10 1 / T A = log10 100 / %T A = 2 - log10 %T QUESTION : WHY DO WE PREFER TO EXPRESS THE BEER-LAMBERT LAW USING ABSORBANCE AS A MEASURE OF THE ABSORPTION RATHER THAN %T ? Compare the two equations that we use: A=abc %T = 100 P/P0 = e -abc COMPARE HOW EACH EQUATION GRAPHS BEER’S LAW EQUATION A=abc Where, a= molar absorptivity (is a measure of the amount of light absorbed per unit concentration; this value is a constant for a given solution) b = cell path length (usually 1cm) (Cuvette) c = concentration (M) BEER’S LAW A = abc Where ab = constant, then A = constant times c So when we plot this y=mx+b BEER’S LAW In order to use A = abc, we need to define values for a and b. b = path length – “blank” – distilled water in a cuvette a = colorimeter(select a preset wavelength) spectrophotometer (choose wavelength where maximum absorption of photons occurs)