BIO103 Experiment 3

EXPERIMENT 3
SPECTROPHOTOMETRIC MEASUREMENT OF
ABSORPTION
Name and Surname :___________________________
Lab Section
Date
:___________________________
:___________________________
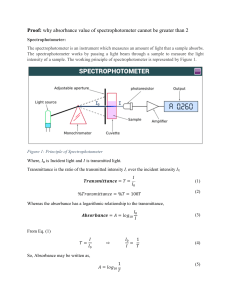
1. INTRODUCTION AND AIM
What is the aim of using a spectrophotometer in biological eperiments?
What are the basic components of a spectrophotometer? Describe their functions.
Please define transmittance (T) and absorption (A) in your own words.
Which property of a solution affects its absorbance value? Explain.
1
2. MATERIALS AND METHODS
Part 1:
How did you operate the spectrophotometer?
Describe the protocol you have used to determine the wavelength of maximum absorbance by bromophenol blue.
Part 2:
Describe the protocol you have used to demonstrate the linear relationship between absorbance and concentration.
How did you determine the concentration of bromophenol blue in the unknown solution?
2
3. RESULTS
Part1:
Record the absorbance values (OD) of the 0.02 mg/ml dye solution for 475-675 nm interval in the following table.
Wavelenght
(nm)
Absorbance
(OD)
Plot these values on a graph (independent variable should be on the X-axis).
What is the wavelength of maximum absorbance?
Part 2:
Record your absorbance values to the table below.
Concentration
(mg/ml)
Absorbance
Plot these values on a graph.
What is the concentration of the dye in the unknown solution?
3
4. DISCUSSION:
Evaluate your results for part I.
Evaluate your results for part II.
Write a short paragraph describing what you have learned in this lab session.
4