330.16.01a.Thermochemistry Calorimetry.Intro
advertisement

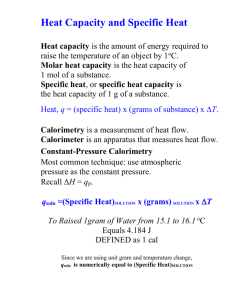
Thermochemistry • The study and measurement of the heat involed or absorbed during chemical reactions. • Heat Temperature – Temperature = average kinetic energy – which does depend on heat, but is not the same Energy Heat vs. Temperature • Heat = Energy that flows between two samples of matter due to differences in temperature. • ¿Which has more heat? – a cup of coffee or a bathtub filled with coffee both at 170 oF? (which takes longer to heat up?) ¿Carpet or hard wood floor in the same room? Energy Calorimetry • Calorimetry: • calor (L) + metry (Gr) • The measurement of heat changes. Calorimetry Calorimetry • Essential Terms: • Specific Heat (s) amount of heat required to raise the temperature of 1 gram of substance by 1oC. intensive property • The higher the specific heat, the more energy it takes to heat up the sample. And, the slower it loses heat. • (N.B., Temperature = average kinetic energy is affected by heat. But also includes potential energy (e.g., the chemical bonds broke when wood burns Calorimetry Calorimetry • Essential Equations: • Specific Heat (s) q = m s DT • m = mass (g) s = specific heat ( amt. heat required to raise the temperature of 1 gram of a substance by 1oC (J/g oC) DT = change in temperature (oC) q = heat associated with reaction (‘ – q’ = exothermic ‘+ q’ = endothermic) •‘ SI Units: Calorimetry Joule(J) 1 J = 4.184 calories food Calorie is 1,000 calories Calorimetry Two Types of Calorimeters: • Constant-Volume Calorimeter • used for combustion reaction (e.g., Calories in food) • Constant-Pressure Calorimeter • used for other reactions (e.g., heat pack: CaCl2 + H2O) we use this in class Calorimetry • Specific Heat & Temperature of a Single Substance Example 6.5 A 394-g sample of water is heated from 10.75°C to 83.20°C. Calculate the amount of heat absorbed (in kilojoules) by the water. Solution q m s DT J 1kJ o q = (394g)(4.184 o )(83.20 -10.75 C)( ) g× C 1000J q =119kJ Check Sign (+) means heat was absorbed; water was heated; Reaction was endothermic so ‘q’ should be ‘+’ Calorimetry • Measuring Heat Change: qsys = qcal + qrxn = 0 (conservation of energy) qcal = heat capacity of calorimeter (assume all q is transferred to water) qrxn = –qcal mA sA DTA = –mB sB DTB Calorimetry • Constant-Pressure Calorimetry Example 6.7 A lead (Pb) pellet having a mass of 26.47 g at 89.98°C was placed in a constant-pressure calorimeter of negligible heat capacity containing 100.0 mL of water. The water temperature rose from 22.50°C to 23.17°C. What is the specific heat of the lead pellet? Strategy Draw the initial and conditions. (It really does help!) Calorimetry • Constant-Pressure Calorimetry Summary Strategy Treat the calorimeter as an isolated system. We know the masses of water and the lead pellet as well as the initial and final temperatures. Assuming no heat is lost to the surroundings, we can equate the heat lost by the lead pellet to the heat gained by the water. Knowing the specific heat of water, we can then calculate the specific heat of lead. m s DT = – m s DT Pb Pb Calorimetry Pb H2O H2O H2O • Constant-Pressure Calorimetry Solution Calorimetry Fin Calorimetry