Q = MC*T * Specific Heat - OISE-IS-Chemistry-2011-2012
advertisement
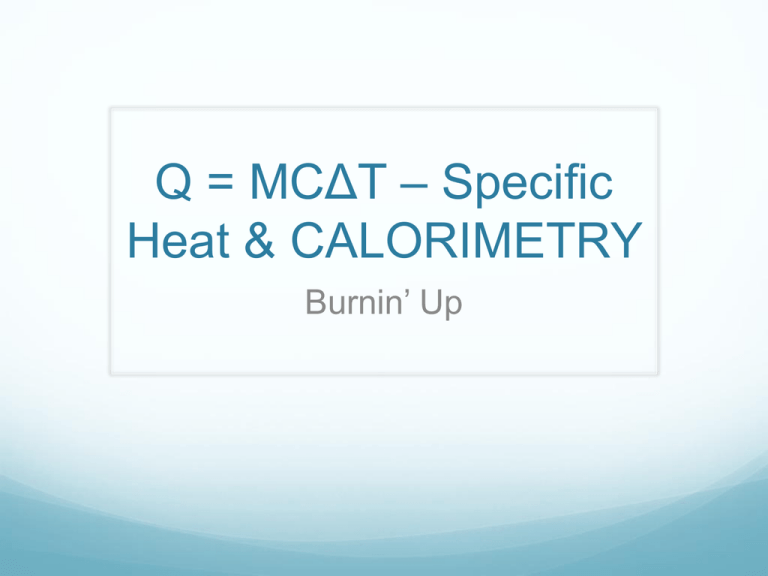
Q = MCΔT – Specific Heat & CALORIMETRY Burnin’ Up What is heat? Thermal energy (kinetic) produced by the movement of molecules Why might we want to measure heat? We’re going camping… I can only take 1L of gas. Should I take PROPANE GAS? or BUTANE GAS? How might I decide? How about another example… How many calories are in our food? What are ‘calories’ in food? How much ‘energy’ you will gain if you eat it How much heat will be produced if we burn it How do we determine the caloric content of food? BURN IT!!! Nowadays they use more sophisticated methods to determine the components Proteins 4 cal/g Carbohydrates 4 cal/g Fats 9 cal/g It’s Experiment Time! VS Comparing it CHEETOS CHIPS 550cal for 95 grams 390cal for 75 grams = 5.78cal/gram = 5.2cal/gram x 4.184KJ/cal x 4.184KJ/cal = 24.18 KJ/gram =21.76kJ/gram We’ve been talking about Q=MC(delta)T What is Calorimetry? the technological process of measuring energy changes in a chemical system What is a Calorimeter? A device used to measure the energy change of a chemical system Used to insulate the reaction against energy/product loss Examples of Calorimeters Bomb Calorimeter Often used for combustion reactions (not necessarily for ‘bombs’!) Calorimetry Questions Question 1: Together It takes 41.8J to heat a 0.01869kg chunk of gold from 10oC to 27oC. Calculate the specific heat capacity of gold. Steps we followed Six Steps of Preparation Four Steps of Action 1. What do we need to find? 7. Sub in the numbers 2. What numbers do we have? 8. Do the math 3. What ‘hidden numbers’ do we have? 4. What equation(s) can we use? 5. Do we need to rearrange the equation? 6. Do we need to convert any units? – WITH UNITS! -- WITH SIGNS! (+/-) 9. Round the answer to the appropriate significant digits 10. Check your answer: Does it make sense? Does it answer the question that was being asked? Question 2: On your own On a mountaineering expedition, a climber heats water from 0oC to 50oC. Calculate the mass of water that could be warmed by the addition of 8.00kJ of heat. Let’s take it one step further I took a 0.400kg block of Aluminum, and heated it on a hot plate to 212oC. I then transferred it to 0.800L of water at 23.0oC. The final temperature of the system was 41.35oC. What is the specific heat capacity of aluminum? Homework Finish pre-lab for NEXT CLASS (Friday) No pre-lab = no lab Calorimetry worksheet is due on TUESDAY Look over ‘cheat sheet’ – it may help you understand the worksheet