Molecular Shapes and Polarity
advertisement
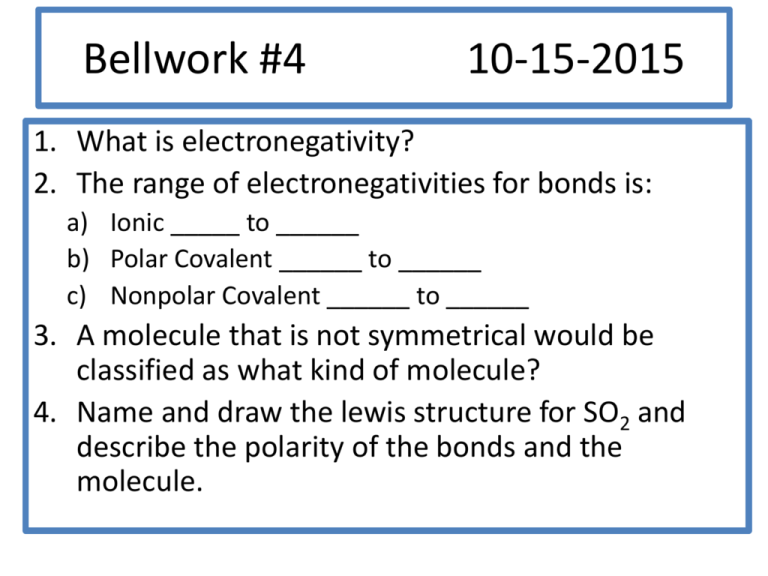
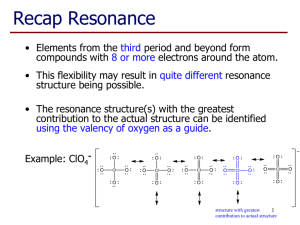
Bellwork #4 10-15-2015 1. What is electronegativity? 2. The range of electronegativities for bonds is: a) Ionic _____ to ______ b) Polar Covalent ______ to ______ c) Nonpolar Covalent ______ to ______ 3. A molecule that is not symmetrical would be classified as what kind of molecule? 4. Name and draw the lewis structure for SO2 and describe the polarity of the bonds and the molecule. Bellwork #4 10-15-2015 Please put your take-home quiz on the front bench! Pick up a Shape Name Inquiry from the bench! 1. What does VSEPR stand for? 2. What is VSPER Theory? 3. How do you think electrons are responsible for the shape of a molecule? Chemical Bonding: Molecular Geometry How is molecular geometry determined? With VSEPR Theory! VSEPR Theory is the idea that electron groups repel one another. V = Valence S = Shell E = Electron P = Pair R = Repulsion What is an electron group? An electron group can be defined as a lone pair, single bond, double bond, or triple bond. Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the electron (bonding and nonbonding) pairs. # of Electron # of Bonding # lone Groups on Groups around pairs on central atom central atom central atom 2 2 Arrangement of electron pairs 0 Molecular Geometry linear linear B B B B Example of LINEAR Molecular Geometry 0 lone pairs on central atom Cl Be Cl 2 electron groups on central atom VSEPR # of Bonding # lone # of Electron Groups around pairs on central atom Groups central atom 3 3 0 3 2 1 Lone pair of electrons repels the electrons in the bond and pushes them down. Arrangement of electron pairs trigonal planar Molecular Geometry trigonal planar Trigonal planar Bent S O O Example of TRIGONAL PLANAR Molecular Geometry VSEPR # of Bonding # lone # of Electron Groups around pairs on central atom Groups central atom 4 4 4 4 3 2 0 Arrangement of electron pairs tetrahedral tetrahedral tetrahedral trigonal pyramidal tetrahedral bent 1 2 Molecular Geometry lone-pair vs. bonding lone-pair vs. lone pair bonding-pair vs. bonding < < pair repulsion repulsion pair repulsion Predicting Molecular Geometry 1. Draw Lewis structure for molecule. 2. Count number of electron groups on the central atom. Compare the bonding groups to the lone pairs. 3. Use VSEPR to predict the geometry of the molecule. What are the molecular geometries of SO2 and SO3? O S Bent O O O S O Trigonal Planar