molecular geometry
advertisement
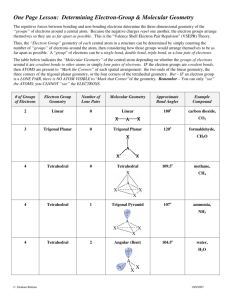
Chemical Bonding and Molecular Geometry Valence-Shell Electron-Pair Repulsion (VSEPR) Theory - Electron pairs repel each other, both bond pairs and unshared (lone pairs) - Electron pairs assume orientations that minimize repulsions. Applying VSEPR Theory 1. Draw a Lewis structure 2. Determine the number of electron groups around the central atom and identify them as being either bond pairs or lone pairs. 3. Establish the electron group geometry around the central atom-linear,trigonal-planar,tetrahedral 4. Determine the molecular geometry from the positions around the central atom occupied by the other atomic nuclei. Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the repulsions between the electron (bonding and nonbonding) pairs. # of atoms bonded to central atom 2 # lone pairs on central atom Arrangement of electron pairs Molecular Geometry 0 linear linear B B 10.1 Cl Be Cl 0 lone pairs on central atom 2 atoms bonded to central atom 10.1 VSEPR # of atoms bonded to central atom 2 3 # lone pairs on central atom Arrangement of electron pairs Molecular Geometry 0 linear linear 0 trigonal planar trigonal planar 10.1 10.1 VSEPR # of atoms bonded to central atom # lone pairs on central atom 3 0 2 1 Arrangement of electron pairs Molecular Geometry trigonal planar trigonal planar trigonal planar bent 10.1 VSEPR # of atoms bonded to central atom # lone pairs on central atom Arrangement of electron pairs Molecular Geometry 2 0 linear linear 3 0 trigonal planar trigonal planar 4 0 tetrahedral tetrahedral 10.1 10.1 VSEPR # of atoms bonded to central atom # lone pairs on central atom 4 0 3 1 Arrangement of electron pairs Molecular Geometry tetrahedral tetrahedral tetrahedral trigonal pyramidal 10.1 10.1 Generic Formula Hybrid Atomic Orbitals (1931 - Linus Pauling) •Proposed that the outermost (valence) orbitals of an atom could be combined to form hybrid atomic orbitals. Sigma bond ( The end-to-end overlapping of an s orbital with a p orbital to form a sp hybrid orbital. Pi bond ( ) - The side-to-side overlapping of two p orbitals. •Single bonds are made up of one sigma bond. •Double bonds are made up of one sigma bond and one pi bond. •Triple bonds are made up of one sigma bond and two pi bonds. Hybridization - A mixture of two or more atomic orbitals. Numbe of Place Where Electron are Found MX 1 MX2 2 MX3 3 MX2E 3 MX4 4 MX3E 4 MX2E2 4 MX5 5 MX4E 5 MX3E2 5 MX2E3 5 MX6 6 MX5E 6 MX4E 6