pptx
advertisement
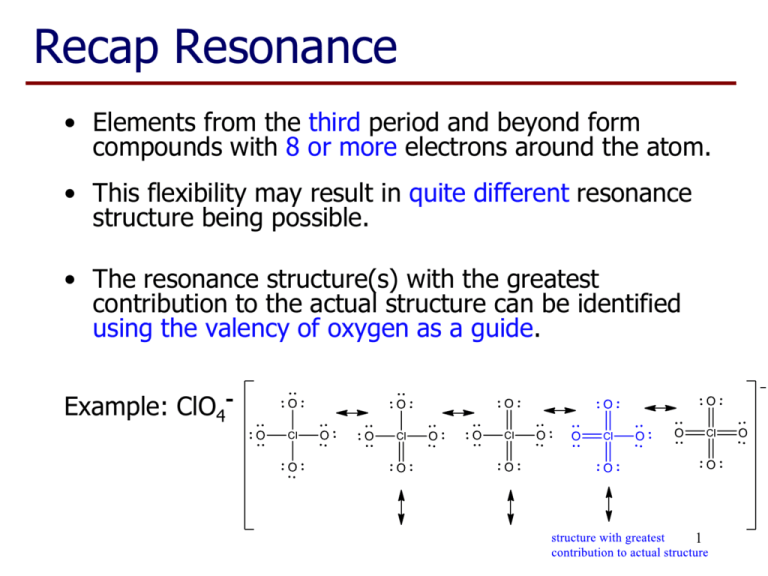
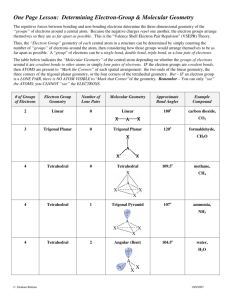
Recap Resonance • Elements from the third period and beyond form compounds with 8 or more electrons around the atom. • This flexibility may result in quite different resonance structure being possible. • The resonance structure(s) with the greatest contribution to the actual structure can be identified using the valency of oxygen as a guide. Example: ClO4- 1 VSEPR Theory Lewis structures give bonding arrangements but do not imply any molecular shape. For this we use: Valence Shell Electron Pair Repulsion Theory This relies on minimising repulsion between areas of electrons (bond pairs and lone pairs) around the central atom. 2 VSEPR Theory 1. Draw Lewis Structure. 2. Count number of electron pairs. • • Count both bonding pairs and non-bonding pairs. Count multiple bonds as only one area of electrons. 3. Determine the arrangement of electron pairs. • Electron pairs want to be as far away from each other as possible. 4. Use atom positions to name molecular geometry. • This is the atom positions. 3 Electron Pair Arrangements • Two electron pairs: – Atoms at the opposite ends of a line. – 180 degrees between areas of electrons. – Called linear. – eg CO2 4 Electron Pair Arrangements • Three electron pairs: – Atoms at the corners of a triangle. – 120 degrees between electron pairs. – Called trigonal planar. – Eg BF3 5 Electron Pair Arrangements • Four electron pairs: – Atoms at the corners of a tetrahedron. – 109.5 degrees between electron pairs. – Called tetrahedral. – Eg CH4 6 Molecular Geometry Remove one arm from the electron pair arrangement for each lone pair present. Trigonal Planar (3 e- pairs) Figure 10.4 Silberberg 7 Molecular Geometry Tetrahedral (4 e- pairs) Figure 10.5 Silberberg 8 Molecular Geometry • Repulsion: lone pair-lone pair > lone pairbond pair > bond pair-bond pair. 109.5 107 104.5 9 Figure 10.9 Silberberg Molecular Geometry 10 Molecular Geometry - Example • Molecules with multiple bonds eg COCl2 total 24 e- ~120 3 areas of electrons about C, so trigonal planar arrangement of electrons No lone pairs so molecular geometry is also trigonal planar 11 Molecular Geometry - Example • Cases when there is no single central atom – Just apply the VSEPR rules to each central atom in turn. ~120 3 areas of electrons about each C, so trigonal planar arrangement of electrons about each C 12 Dipole Moments • Any bond between two different atoms will be polar. • A molecule has a permanent dipole moment if it contains polar bonds and it is not a symmetrical shape. • Note: Cations and anions are not polar – the overall charge overwhelms any local d+ vs deffects. 13 Dipole Moments • Polar molecules HF H2O • Non-polar molecules CHCl3 CCl4 N2 CO2 14 Learning Outcomes: • By the end of this lecture, you should: − work out the number of bonding and non-bonding pairs from the Lewis structure of a molecule − predict the distribution of these pairs around an atom − predict and describe the molecular shape − determine if a permanent dipole exists − be able to complete the worksheet (if you haven’t already done so…) 15 Questions to complete for next lecture: 1. Draw the shapes of the following molecules and ions and give approximate bond angles (a) BH3 (b) NH4+ (c) CS2 (d) CH2O (e) CH3Cl (f) H3O+ 16 Questions to complete for next lecture: 2. What are the approximate C-C-C bond angles in the two molecules below? 3. Are these molecules flat? 17