File
advertisement

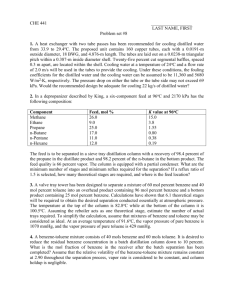
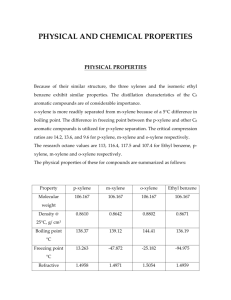
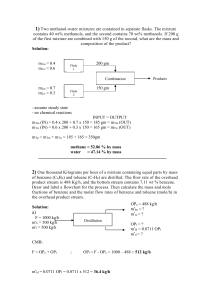
HOMEWORK#3 Due:3-3-14 [1] Table below illustrates chemical and physical properties of some organic compounds. Heptane Benzene Toluene p-Xylene Chlorobenzene Naphthalene Anthracene Fluoranthene MW (g/mol) 100 78.11 92.1 106.2 112.62 128.2 178.2 202.2 MP (°C) liquid liquid liquid liquid liquid 80 219 107 Sw (mg/L) 0.01 1800 525 200 500 32 0.07 0.265 Density (g/mL) 0.684 0.879 0.866 0.861 Log Kow --2.3 2.69 3.15 2.84 3.3 4.45 4.9 Ks (NaCl) ---0.2 0.2 0.2 [a] Estimate the γw for chlorobenzene, naphthalene, and anthracene using the information in the provided table. [b] Find the solubility of fluoranthene at 50% volume fraction methanol assuming σc=log Kow. [c] Compare your estimated value with measured value. Measured value at 50% volume fraction methanol is 62 mg/L. [2] Consider underground storage tanks buried near a shoreline where saltwater intrusion occurs. Gasoline is leaking from the tanks. Assume gasoline is approximately 60% alkanes, which can be represented by heptane (average between butane, pentane, octane etc.) and the rest is primarily benzene (5 wt% benzene, 22 wt% toluene, 13 wt% p-xylene). Most gasoline has an average molecular weight of 105 g/mole and an average density of 0.74 g/mL. Assume ionic composition and strength of saltwater/groundwater mix in contact with the plume can be approximated with 0.25 M NaCl. Estimate the aqueous phase concentration in mg/L benzene, toluene, and pxylene leaving the gasoline tank. [3] Fedinger and co-workers developed a laboratory method referred to as the “wettedwall column method” for determining air-water Henry’s Law constants for pesticides. This method consists of equilibrating the solute between a thin flowing film of water and concurrent air stream in a vertical column. They reported a dimesionless Henry’s constant for lindane (1,2,3,4,5,6- hexachlorocyclohexane) to be 8.1 x 10-5. If lindane crystals are placed in a closed system and the second flask contains distilled water, what will be the activity coefficient of lindane in the aqueous phase be after sufficient amount of time has elapsed to ensure equilibration. [4] Estimate KH (in atm L/mol) and KH’ (dimesionless) at 25 °C for [a] chlorobenzene and [b] naphthalene using data provided above. [5] Calculate the equilibrium distribution ratio, KH,SW of fluorine between air and seawater at 25 °C. [6] List the chemicals given in Table below in terms of increasing Henry’s constant (K H) (e.g., A>B>C with A having the largest Henry’s Law constant. Chemical Naphthalene p-Xylene Hexane Tetrachloroethane MW (g/mol) 128 106 86 166 Tb (°C) 218 138.1 69 121.1 Tm (°C) 80.2 13.3 -95 -22.4 Sw (mol/L) 2.5x10-4 1.8x20-3 1.4x10-4 0.025 [7] List the chamicals listed in Table below in terms of increasing aqueous solubility (S w). Chemcial Benzene Ethanol Toluene Phenanthrene MW (g/mol) 78 46 92 178 Tb (°C) liquid liquid liquid 100 Tm (°C) 80.1 78 111 340