HW 12
advertisement

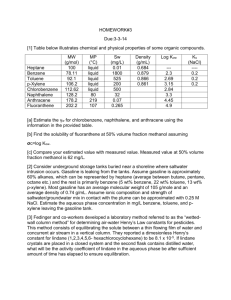
HW #12‐CHEN 354‐Due Friday, April 23, 2010 Problem 1. A 50 wt% (22.5 mol%) solution of ethylene‐glycol and water freezes at 240 K. What freezing temperature would be predicted by assuming that ethylene glycol and water form an ideal solution? The freezing occurs by formation of water crystals. o Problem 2. Estimate the solubility of naphthalene (1) in nitrogen (2) at 35 C at pressures up to 300 bar. Use the procedure in section 14.7 with l12 =0, and l12 =0.05 . Compare the results with those in Fig. 14.23 o for naphthalene/CO2 at 35 C with l12 =0 and l12 =0.088. Discuss any differences. Problem 3. A liquid mixture of benzene and n‐heptane is to be cooled to a temperature as low as possible without precipitation of a solid phase. If the solution contains 10 mol% benzene, estimate what this lowest temperature is. Solids are immiscible. Data is as follows: Property melting point (K) molar liquid volume (cm3/mol) Enthalpy of fusion (J/mol) Molar liquid volume (cm3/mol) Benzene 278.7 89 9843 89 n‐Heptane 182.6 148 14067 148 In this system the activity coefficients in the liquid phase are given by: where 2 is the volume fraction of component 2, and Problem 4. A liquid mixture contains 5mol% naphthalene and 95 mol % benzene. The mixture is slowly cooled at constant pressure. At what temperature does a solid phase appear? Assume ideal mixing in the liquid phase and total immiscibility in the solid phase. Data are: Property Melting temperature (K) Enthalpy of fusion (J/mol) benzene 278.7 9843 naphthalene 353.4 19008