word - Seattle Central College
advertisement

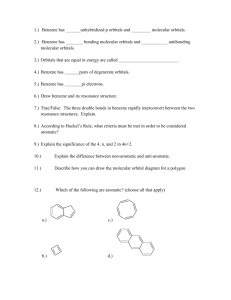
CHEM 122: Introduction to Organic Chemistry Chapter 4: Benzene and its Derivatives. 1. Draw at least two structural formulas for each of the following. (Several constitutional isomers are possible for each part.) a) b) c) d) An alkene of six carbons A cycloalkane of six carbons An alkyne of six carbons An aromatic hydrocarbon of eight carbons 2. Account for the fact that the six-membered ring in benzene is planar but the sixmembered ring in cyclohexane is not. 3. The compound 1,4-dichlorobenzene (p-dichlorobenzene) has a rigid geometry that does not allow for free rotation. Yet no cis-trans isomers exist for this structure. Explain why it does not show cis-trans isomerism. 4. Name these compounds. NO 2 CH3 a) Cl Cl b) c) C6H5CH2CH2CH2Cl d) C6H5CCH2CH3 CH3 CH3 NH 2 OH NO 2 e) Cl C6 H5 f) g) C=C H h) C6H5 Cl 5. Draw structural formulas for these compounds. a) b) c) d) e) f) 1-Bromo-2-chloro-4-ethylbenzene 4-Bromo-1,2-dimethylbenzene 2,4,6-Trinitrotoluene 4-Phenyl-1-pentene p-Cresol 2,4-Dichlorophenol 6. Following is the structural formula of styrene, the monomer from which the polymer polystyrene is prepared. CH=CH 2 polymerization Polystyrene Styrene Draw a section of a polystyrene chain showing at least three monomer units. 7. Suppose you have unlabeled bottles of benzene and cyclohexene. What chemical reaction could you use to tell which bottle contains which chemical? Explain what you would do, what you would expect to see, and how you would explain your observations. Write an equation for a positive test. 8. Two products with the molecular formula C6H4BrCl form when bromobenzene is treated with chlorine, Cl2, in the presence of FeCl3 as a catalyst. Name and draw a structural formula for each product. 9. What reagents and/or catalyst are necessary to carry out each conversion? a) Benzene to nitrobenzene b) 1,4-dichlorobenzene to 2-bromo-1,4-dichlorobenzene c) Benzene to aniline 10. What reagents and/or catalyst are necessary to carry out each conversion? Each conversion requires two steps. a) Benzene to 3-nitrobenzenesulfonic acid b) Benzene to 1-bromo-4-chlorobenzene 11. Aromatic substitution can be done on naphthalene. Treatment of naphthalene with concentrated H2SO4 gives two (and only two) different sulfonic acids. Draw a structural formula for each. 12. Both phenol and cyclohexanol are only slightly soluble in water. Account for the fact that phenol dissolves in aqueous sodium hydroxide but cyclohexanol does not. 13. The structure for naphthalene given below is only one of three possible resonance structures. Draw the other two. Naphthalene 14. Draw structural formulas for these compounds. a) b) c) d) e) f) 1-Phenylcyclopropanol Styrene m-Bromophenol 4-Nitrobenzoic acid Isobutylbenzene m-Xylene 15. Write the structural formula for the product of each reaction. HNO3 H2SO4 a) CH3 Br2 FeBr3 CH3 b) Br c) H2SO4 Br 16. Styrene reacts with bromine to give a compound with the molecular formula C8H8Br2. Draw a structural formula for this compound.