tutorial 2 - distillation
advertisement

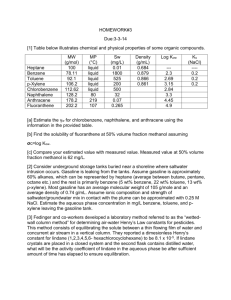
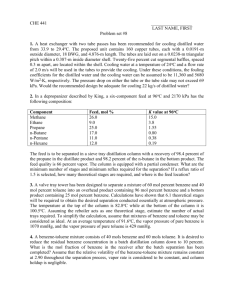
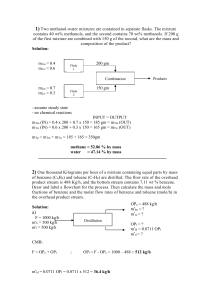
ERT 313 BIOSEPARATION ENGINEERING TUTORIAL 2 - DISTILLATION Prepared by: Miss Hairul Nazirah Abdul Halim QUESTION 1 – EXAMPLE 21.2 (McCABE) A continuous fractionating column is to be designed to separate 30,000 kg/h of a mixture of 40 percent benzene and 60 percent toluene into an overhead product containing 97 percent benzene and a bottom product containing 98 percent toluene. These percentages are by weight. A reflux ratio of 3.5 mol to 1 mol of product is to be used. The molal latent heats of benzene and toluene are 7,360 and 7,960 cal/g mol, respectively. Benzene and toluene form a nearly ideal system with a relative volatility of about 2.5; the equilibrium data is shown in Table 11.1-1. The feed has a boiling point of 95°C at a pressure of 1 atm. (a) Calculate the moles of overhead product and bottom product per hour. (a) Determine the number of ideal plates and the position of the feed plate (i) if the feed is liquid and at its boiling point; (ii) if the feed is liquid and at 20°C (specific heat 0.44 cal/g.°C); (iii) if the feed is a mixture of two-thirds vapor and one- third liquid. EXAMPLE 11.4-3. (Geankoplis) Number of Trays in Stripping Tower *Feed enter at the top of the column, no rectifying section A liquid feed at the boiling point of 400 kg mol/h containing 70 mol % benzene (A) and 30 mol % toluene (B) is fed to a stripping tower at 101.3 kPa pressure. The bottoms product flow is to be 60 kg mol/h containing only 10 mol % A and the rest B. Calculate the kg mol/h overhead vapor, its composition, and the number of theoretical steps required. Ans: D = 340 kgmol/h, XD = 0.806, 4.6 theoretical trays + a reboiler EXAMPLE 11.4-1 (Geankoplis). Rectification of a Benzene - Toluene Mixture A liquid mixture of benzene - toluene is to be distilled in a fractionating tower at 101.3 kPa pressure. The feed of 100 kg mol/h is liquid, containing 45 mol % benzene and 55 mol % toluene, and enters at 327.6 K. A distillate containing 95 mol % benzene and 5 mol % toluene and a bottoms containing 10 mol % benzene and 90 mol % toluene are to be obtained. The reflux ratio is 4:1. The average heat capacity of the feed is 159 kJ/kg mol.K and the average latent heat 32099 kJ/kg mol). Equilibrium data for this system are given in Table 11.1-1. Calculate the kg moles per hour distillate, kg moles per hour bottoms, and the number of theoretical trays needed. *b.p of benzene = 366.7 K Ans: D = 41.18 kgmol/h, B = 58.82 kgmol/h, 6.6 theoretical trays + a reboiler, feed plate: plate 5 from top