Test Recovery packet_ bonding
advertisement
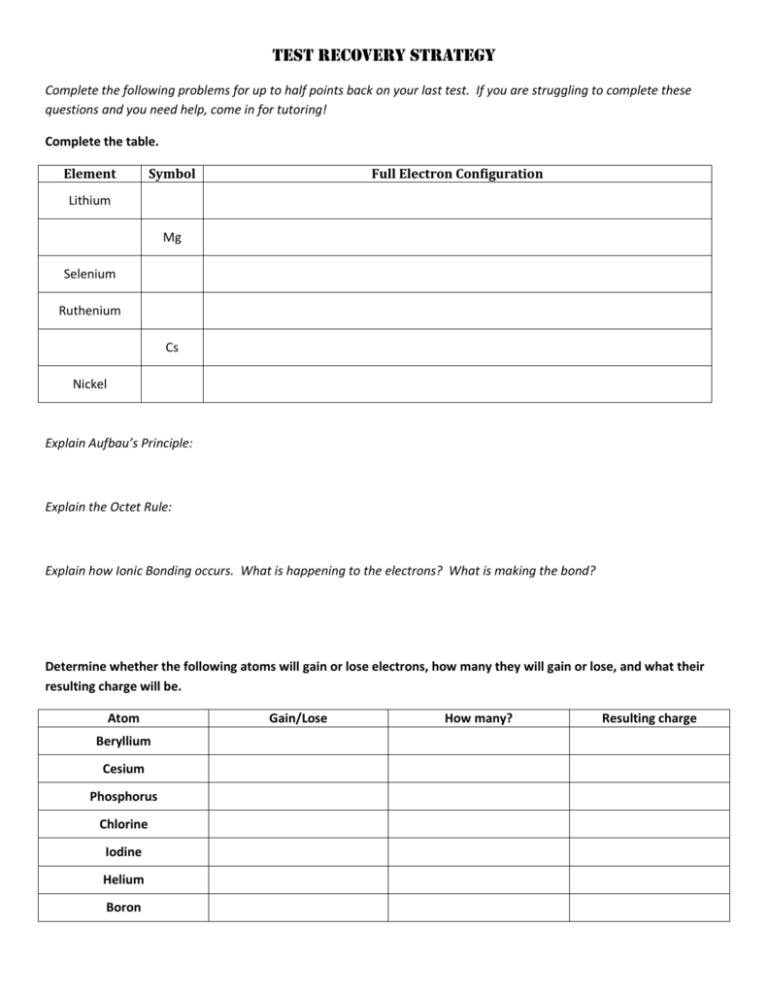
Test Recovery Strategy Complete the following problems for up to half points back on your last test. If you are struggling to complete these questions and you need help, come in for tutoring! Complete the table. Element Symbol Full Electron Configuration Lithium Mg Selenium Ruthenium Cs Nickel Explain Aufbau’s Principle: Explain the Octet Rule: Explain how Ionic Bonding occurs. What is happening to the electrons? What is making the bond? Determine whether the following atoms will gain or lose electrons, how many they will gain or lose, and what their resulting charge will be. Atom Beryllium Cesium Phosphorus Chlorine Iodine Helium Boron Gain/Lose How many? Resulting charge Determine what types of bonds are formed between the following atoms. (Ionic, Polar covalent, Non-polar covalent) and indicate what is happening to their electrons. (Shared equally, Shared unequally, Transferred) Atoms Math Type of Bond Electrons Boron and Oxygen Lithium and Chlorine Carbon and Hydrogen Hydrogen and Hydrogen Cesium and Bromine Silicon and Phosphorus Identify each of the following properties as belonging to an Ionic (I), Covalent (C), or Metallic (M) compound. ____ Forms a crystal lattice structure ____ Is difficult to crush ____ Is easy to crush ____ Has a high melting point ____ Is easy to melt ____ Salt ____ Conducts electricity as a solid and a liquid ____ Is a liquid or gas at room temperature ____ Conducts electricity as a liquid ____ Has a “sea of electrons” For the following formulas, draw a Lewis Structure, determine the shape of the molecule based on VSEPR theory, and identify whether the molecule is polar or non-polar. 1) PBr3 Shape: ______________________ Polar/Non-polar 2) N2H2 Shape: _____________________ Polar/Non-polar 3) NO2-1 Shape: ______________________ Polar/Non-polar 4) C2H4 Shape: _________________________ Polar/Non-polar 5) CCl4 Shape: ________________________ Polar/Non-polar 6) BH3 Shape: ________________________ Polar/Non-polar 7) SiS2 Shape: ________________________ Polar/Non-polar 8) C2H2 Shape: ________________________ Polar/Non-polar 9) PF3 Shape: _________________________ Polar/Non-polar Explain why water is classified as a POLAR COVALENT molecule in terms of electronegativity, bond polarity and molecular polarity.









