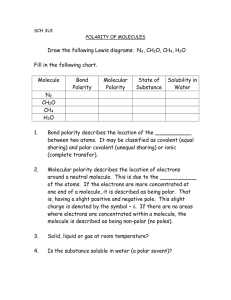
Molecule Worksheet 3 Complete the Following Table Include partial
advertisement

Molecule Worksheet 3 Complete the Following Table Include partial pos and partial neg with dipole moment, Draw with correct VSEPR 3D structures, Identify molecular polarity, Identify whether the molecule would create an aqueous solution Will it Molecule Lewis Dot Polarity Dissolve in: H2O CCl4 CHCl3 CH4CO2 CH3CH2OH CH3NH2 CH3(CH2)2OH CH3COOH Polarity Multiple Choice, Circle the best answer 1. Why is H2O a polar molecule? a. It will freeze at polar temperatures b. Since Hydrogen is polar and so is Oxygen, then the combination is also polar c. The Oxygen bonds with the Hydrogen electrons, causing the (+) Hydrogen nucleus to be exposed at one end 2. Why don't the electrons give a non-polar molecule charges at their ends? a. Charges from the protons cancel out charges from the electrons b. Non-polar molecules don't have charged “ends” c. Electrons don't have any charge 3. What happens if you mix two liquids, where each consists of polar molecules, and the liquids don't react chemically? a. The combination turns to a solid substance b. The combination becomes a solution c. Such liquids will not mix Label the hybridization (sp, sp2, sp3) for each carbon for the following organic molecules: Draw the QM model orbital diagrams for 3 of the molecules listed above in the provided space. Be sure to label each atom and bond. Place a number in the box above to correlate with the molecule you chose to draw. 1. 2. 3.