Polar Molecules Independent Work
advertisement

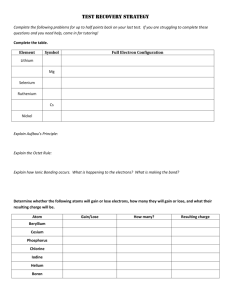
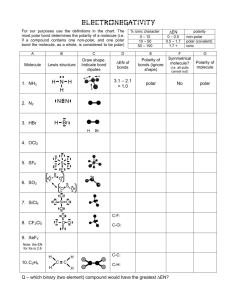
Polar Molecules Part A – Read Section 4.4 of your textbook. Answer the following questions as you read: 1. What is a polar molecule? 2. What is the difference between a polar molecule and a charged molecule (or ion)? 3. Define electronegativity. 4. Describe the trends of electronegativity (increasing/decreasing) across periods (horizontal) and groups (vertical) on the periodic table. 5. Explain how to determine whether a chemical bond is non-polar covalent, polar covalent, or ionic. 6. Explain the meaning of this statement – “The difference between a polar covalent bond and an ionic bond is in shades of grey, rather than black or white.” 7. For each of the following, label the electronegativities. Label any partial charges and classify as ionic, polar covalent, or non-polar covalent. Electronegativity of atoms can be found on the periodic table at the back of your text. a. H – Cl c. N – O e. Mg – S b. C – H d. I – Br f. P – H 8. Explain the difference between a polar bond and a polar molecule. 9. Explain why carbon tetrafluoride (CF4) is a non-polar molecule, even though it possesses polar bonds. 10. Predict whether the following molecules are polar or non-polar. Include the VSEPR diagram of the molecule and bond dipoles to make an accurate prediction. a. Boron trifluoride, BF3 f. Ethene, C2H4 b. Oxygen difluoride, OF2 g. chloroethane, C2H5Cl c. Carbon tetraiodide, CI4 h. Methylamine, CH3NH2 d. Phosphorous trichloride, PCl3 i. Ethanol, C2H5OH e. Dichlorofluoroethane, CHFCl2 j. Diboron tetrafluoride, B2F4 **Answers are posted on our website** Part B Finish your VSEPR and Polarity Assignment by stating whether or not each molecule is polar or nonpolar.