Bio 101 Study Guide Exam 1
advertisement

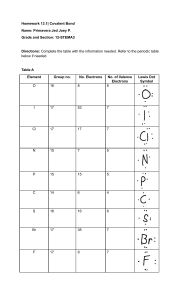
Bio 101 Study Objectives for Exam 1 1. Know the characteristics of life 2. Understand how transfer of energy in an ecosystem works between the trophic levels 3. Know two basic cell types 4. Understand the basic steps of the scientific method and the proper design of an experiment. Including the statement of the hypothesis and use of controls. Definitions Energy Matter Producers Consumers/ decomposers Hypothesis 1. Given any element and a periodic chart be able to determine the number of protons, neutrons and electrons, the atomic number and atomic weight, the number of valence electrons and the valence (missing number in valence shell) 2. Definitions of a. element b. compound c. trace elements d. isotope e. ion f. isomer (don’t get d, e and f mixed up) g. proton h. neutron i. electron j. anion k. cation 3. Understand how covalent bonds form and the difference between polar covalent and non-polar covalent 4. How does an ionic bond form? 5. What is a H bond and know an example. 6. What are Van der Waals interactions? 7. About chemical reactions: a. What is a chemical reation? b. Reactants c. Products 8. The polar nature of water gives it extraordinary characteristics” a. Cohesion versus adhesion b. Moderation of temperature i. Specific heat ii. Evaporation iii. Know the difference between temperature and heat c. Why does ice float and what is the significance of this? d. Solvent of life polar and ionic substances i. Solvent ii. Solute iii. Solution 9. Definition of (what does it do when added to a solution?) a. Acid b. Base 10. pH range for acid and base- know how a change in pH changes the concentration of hydrogen ions _____times (you fill in the blank). 11. Definition of hydrocarbon 12. Functional groups a. Identify the formula b. Name of the group 13. What are the 4 classes of macromolecules? a. Know the monomer, polymer and bond name for each type b. Polymers are formed through dehydration and broken down by hydrolysis c. Lipids are the only macromolecule not formed like this 14. Carbohydrates a. Disaccharides: monosaccharides that make sucrose and maltose b. Storage polysaccharides: starch and glycogen c. Structural polysaccharides: cellulose and chitin 15. Lipids- fats, lipids, steroids a. Not polymers b. Non-polar c. Fats: glycerol + fatty acid chains i. Saturated ii. Unsaturated d. Phospholipids: glycerol + two fatty acids and a phosphate group e. Steroids: 3 six carbon rings + 1 five carbon ring i. Ex: steroid component of animal cell membranes 16. Proteinsa. know functions b. Four levels of protein structure c. What causes proteins to be denatured d. Definition of an enzyme 17. Nucleic acids are a macromolecule, but are so important they will get their own test later.