Ester Synthesis Lab Report: Reactions & Structures

Making Esters
Purpose : To create some esters and write appropriate structural formulas for the reaction.
Background Information
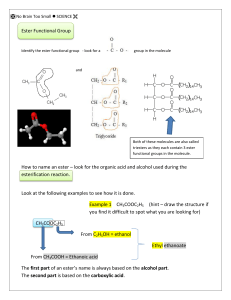
Esters are commonly created by combining a carboxylic acid with an alcohol in the presence of a strong acid. (The hydrogen ions from the acid aid help to break the single bonded oxygen off of the carboxylic acid).
The alcohol loses the hydrogen from its hydroxyl group. This hydrogen atom then combines with the OH from the acid to make water. The remaining portions of the alcohol and acid then combine to form the ester.
A sample ester formation equation.
Materials/Apparatus:
250 ml beaker, hot plate, evaporating dish, test tubes,
1-pentanol, methanol, 1-octanol, ethanoic acid, 2-hydroxybenzoic acid, sulfuric acid (CAUTION--this stuff will burn you!!! Do not allow it to get on your hands or in your eyes!!!)
Procedure:
In general, 1 part of acid and 1 part of alcohol (about 2 ml and 2 ml) are placed in a test tube (in the case of a solid acid, the depth of the solid should be about 1 cm with about 2 ml of alcohol).
(Note: If the methanol dries up, just carefully add some more.)
In order to make the reaction occur, the two compounds must be heated (in the presence of a strong acid – 5 drops) for about 5 minutes at a temperature of about 100 C.
Create about 4 ml of octyl ethanoate, pentyl ethanoate, and methyl 2 hydroxy benzoate.
After heating in a boiling water bath, the product is poured into an evaporating dish which is approximately ½ full of water.
REMEMBER TO “WAFT” when observing odor!!!
Data: Record the aroma of each ester.
Questions:
1. Write structural equations for each of the 3 above reactions. Please refer to the sample ester formation equation in the background info for help. Give the names and structures for all 4 compounds involved in each of these reactions.
2. Write the equation for the reaction of 3-fluorohexanoic acid and 2-propanol to form an ester. Give the names and structures for all 4 compounds involved in this reaction.
3. Write the equation for the formation of propyl 3,4-dimethyl pentanoate. Give the names and structures for all 4 compounds involved in this reaction.
4. Another reaction that involves the release of water in synthesis is the formation of a peptide bond in proteins. (this is called dehydration in biology)
Draw a picture of 2 amino propanoic acid and 2 amino ethanoic acid.
Assume that the OH from the carboxyl group of the 2 amino propanoic acid and one of the H’s from the amine group of the 2 amino ethanoic acid are lost to form water. Draw a picture of the resulting molecule
(which is called a dipeptide), and of water.
NOW, build each molecule with the model kits.
Include the drawings in your lab report. You should have diagrams and pictures for 6 reactions.
Lab Guidelines: (1,2,3,4,5&6[attach handout],7,10)