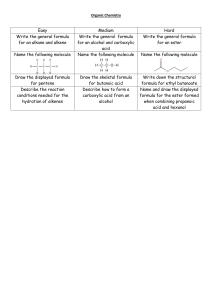
ESTERS STRUCTURE OF ESTERS PREPARATION OF ESTERS CYCLIC ESTERS Escamillan | Estember | Gabinete | Gabunal | Guevarra | Juesna STRUCTURE OF ESTERS Ester is a carboxylic acid derivative in which the -OH portion of the carboxyl group has been replaced with an -OR group. The ester functional group is thus: In linear form, the ester functional group can be represented as -COOR or -COR 2 General formula of Ester RCOOR′ NOTE: R may be a hydrogen atom, an alkyl group, or an aryl group, and R′ may be an alkyl group or an aryl group but not a hydrogen atom The Simplest Ester has a hydrogen atom attached to the ester functional group The structure of the simplest aromatic ester is derived from the structure of the simplest aromatic carboxylic acid Benzoic Acid NOTE: The difference between a carboxylic acid and an ester is a “H versus R” relationship Example of carboxylic acid: - Acetic acid Example of Ester : -Methyl Acetate Structures of carboxylic acids to Esters PREPARATION OF ESTERS Ester is produced through esterification. An esterification reaction is the reaction of a carboxylic acid with an alchohol (or phenol) to produce an ester. A strong acid catalyst (generally H2SO4) is needed for esterification. In the esterification process, a -OH group is lost from the carboxylic acid, a -H atom is lost from the alcohol, and water is formed as a by-product. The net effect of this reaction is substitution of the -OR group of the alcohol for the -OH group of the acid. An example of esterification is the reaction of acetic acid with methyl alcohol. Esterification reactions are equilibrium processes, Usually favoring products only slightly. That is, at equilibrium, substantial amount of both reactants and products are present. The amount of ester formed can be increased by using an excess of alcohol or by constantly removing one of the products. According to Le Chatelier’s principle, either of these techniques will shift the position of equilibrium to the right (the product side of the equation). This equilibrium problem explains the use of the “double-arrow” in all the esterification equations in this section. It is often useful to think of the structure of an ester in terms of its “parent” alcohol and acid molecules; the ester has an acid part and an alcohol part. In this context, it is easy to identify the acid and alcohol from which a given ester can be produced; just add a -OH group to the acid part of an ester and a -H atom to the alcohol part to generate the parent molecules. CYCLIC ESTERS Cyclic Esters (Lactones) Hydroxy acids—compounds which contain both a hydroxyl and a carboxyl group have the capacity to undergo intermolecular esterifi cation to form cyclic esters. Such internal esterifi cation easily takes place in situations where a five- or six-membered ring can be formed. Lactones are formed by intramolecular esterification of the corresponding hydroxycarboxylic acids, which takes place spontaneously when the ring that is formed is five- or six-membered Cyclic esters are formally called lactones. A lactone is a cyclic ester. Like cyclic ethers, cyclic esters are also heterocyclic organic compounds. The oxygen atom that remains after a molecule of water is formed becomes part of the ring structure. Lactones are generally named after the carboxylic acid by using the suffix lactone. A Greek letter is used to indicate the ring size. A lactone with a five-membered ring is a y-lactone because the g-carbon from the carbonyl carbon atom is bonded to the heteroatom (O) of the ring. Similarly, a six-membered lactone ring system is a d-lactone because the d-carbon is bonded to the heteroatom (O) of the ring system. Thank you!