Phenylpropanoids & Tannins: Biosynthesis & Characteristics
advertisement

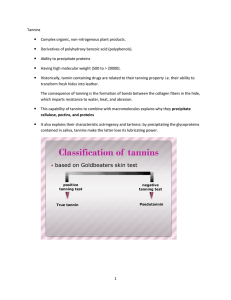
Phenylpropanoids The reaction catalyzed by 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase. The reaction catalyzed by 3-dehydroquinate synthase. The enzyme requires catalytic amounts of NAD for activity. The reaction catalyzed by 3-dehydroquinate dehydratase. The reaction catalyzed by shikimate dedydrogenase. The plant enzyme uses NADP, whereas some microorganisms use pyrrolo quinoline quinone as cofactor. The reaction catalyzed by shikimate kinase. The reaction catalyzed by 5-enolpyruvylshikimate 3-phosphate synthase. The reaction catalyzed by chorismate synthase. The enzyme requires catalytic amounts of reduced flavin for activity. The synthesis of gallic acid, hydroxycinnamat es, stilbenes and flavonoids by the phenylpropanoid pathway in plants Coumarins • • • Coumarin is the parent organic compound of a class of naturally occurring phytochemicals found in many plant species. This oxygen heterocycle is best known for its fragrance, described as a vanilla-like odor or the aroma of freshly mowed hay. Identified in the 1820s, coumarin has been synthesized in the laboratory since 1868 and used to make perfumes and flavorings. It is also used to prepare other chemicals -- in particular anticoagulants and rodent poison. Coumarin is found in a variety of plants such as Tonka bean, lavender, sweet clover grass, and licorice, but also occurs in food plants such as strawberries, apricots, cherries, and cinnamon. It is thought to work by serving as a pesticide for the plants that produce it. Chemically, coumarin can occur either free or combined with the sugar glucose to produce a coumarin glycoside. Medically, coumarin glycosides have been shown to have blood-thinning, anti-fungicidal, and anti-tumor activities. Dicumarol, a coumarin glycoside better known as warfarin, is the most commonly used oral anticoagulant medication. The biosynthesis of shikimate metabolites Andrew R. Knaggs Analytical Sciences Department, Discovery Research, GlaxoSmithKline Medicines Research Centre, Gunnels Wood Road, Stevenage, Hertfordshire, UK SG1 2NY Received (in Cambridge, UK) 20th August 2002 First published as an Advance Article on the web 29th November 2002 Covering 2000. Previous review: Nat. Prod. Rep., 2001, 18, 334 Nat. Prod. Rep., 2003, 20, 119–136 FLAVONOID Biosynthesis Anthocyanin biosynthetic pathway Tannins: definition The word tannin is very old and reflects a traditional technology. "Tanning" (waterproofing and preserving) was the word used to describe the process of transforming animal hides into leather by using plant extracts from different plant parts of different plant species. • Plant parts containing tannins include bark, wood, fruit, fruitpods, leaves, roots, and plant galls. • Examples of plant species used to obtain tannins for tanning purposes are wattle (Acacia sp.), oak (Quercus sp.), eucalyptus (Eucalyptus sp.), birch (Betula sp.), willow (Salix caprea), pine (Pinus sp.), quebracho (Scinopsis balansae) . Tannins: definition Tannins are phenolic compounds that precipitate proteins. They are composed of a very diverse group of oligomers and polymers. There is some confusion about the terminology used to identify or classify a substance as a tannin, In fact, • not only tannins bind and precipitate proteins (other phenolics such as pyrogallol and resorcinol also have this property), • not all polyphenols precipitate proteins or complex with polysaccharides Tannins can complex with: • Proteins • Starch • Cellulose • Minerals . Tannins: interaction with other macromolecules Tannins have a major impact on animal nutrition because of their ability to form complexes with numerous types of molecules, including, but not limited to, • Carbohydrates, • Proteins, • Polysaccharides, • Bacterial cell membranes, • Enzymes involved in protein and carbohydrates digestion. Characteristics of tannins • oligomeric compounds with multiple structure units with free phenolic groups, • molecular weight ranging from 500 to >20,000, • soluble in water, with exception of some high molecular weight structures, • ability to bind proteins and form insoluble or soluble tannin-protein complexes. TANNIN • Condensed Tannin – Epicatechin, Catechin – Procyanidin, Anthocyanidin – Leucoanthocyanidin (thru heat & acid) • Hydrolyzable Tannin – Gallotannin – Egallitannin Hydrolyzable tannins • HTs are molecules with a polyol (generally D-glucose) as a central core. • The hydroxyl groups of these carbohydrates are partially or totally esterified with phenolic groups like gallic acid (-->gallotannins) or ellagic acid (--> ellagitannins). HT are usually present in low amounts in plants. • Some authors define two additional classes of hydrolyzable tannins: taragallotannins(gallic acid and quinic acid as the core) and caffetannins (caffeic acid and quinic acid) Gallotannins • The phenolic groups that esterify with the core are sometimes constituted by dimers or higher oligomers of gallic acid (each single monomer is called galloyl) • Each HT molecule is usually composed of a core of D-glucose and 6 to 9 galloyl groups • In nature, there is abundance of mono and di-galloyl esters of glucose (MW about 900). They are not considered to be tannins. At least 3 hydroxyl groups of the glucose must be esterified to exhibit a sufficiently strong binding capacity to be classified as a tannin. • The most famous source of gallotannins is tannic acid obtained from the twig galls of Rhus semialata. It has a penta galloyl-Dglucose core and five more units of galloyl linked to one of the galloyl of the core. Ellagitannins • The phenolic groups consist of hexahydroxydiphenic acid, which spontaneously dehydrates to the lactone form, ellagic acid. • Molecular weight range: 2000-5000. HT properties: • hydrolyzed by mild acids or mild bases to yield carbohydrate and phenolic acids • Under the same conditions, proanthocyanidins (condensed tannins) do not hydrolyze. • HTs are also hydrolyzed by hot water or enzymes (i.e. tannase). Proanthocyanidins (condensed tannins) • • • • • • • PAs are more often called condensed tannins due to their condensed chemical structure. However, HTs also undergo condensation reaction. The term, condensed tannins, is therefore potentially confusing. The term, proanthocyanidins, is derived from the acid catalyzed oxidation reaction that produces red anthocyanidins upon heating PAs in acidic alcohol solutions. The most common anthocyanidins produced are cyanidin (flavan-3-ol, from procyanidin) and delphinidin (from prodelphinidin) PAs may contain from 2 to 50 or greater flavonoid units; PA polymers have complex structures because the flavonoid units can differ for some substituents and because of the variable sites for interflavan bonds. Anthocyanidin pigments are responsible for the wide array of pink, scarlet, red, mauve, violet, and blue colors in flowers, leaves, fruits, fruit juices, and wines. They are also responsible for the astringent taste of fruit and wines. PA carbon-carbon bonds are not cleaved by hydrolysis. Depending on their chemical structure and degree of polymerization, PAs may or may not be soluble in aqueous organic solvents.