03.Collins.Tannins
advertisement

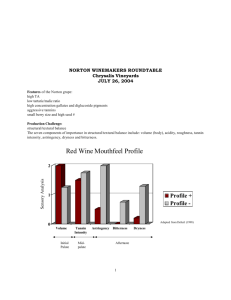
Tannins – types and amounts in grapes and wines Tom Collins Senior Research Enologist Beringer Blass Wine Estates Formerly the Manager of East Coast Grower Relations and Vineyard Operations for Canandaigua Wine Company, Tom Collins is now a Senior Research Enologist with Beringer Blass Wine Estates in St. Helena, CA. His viticultural areas of interest while with Canandaigua included evaluation of new sprayer technology, vineyard mechanization and vineyard redevelopment. In his position with Beringer Blass, Tom oversees the development and implementation of a broad range of winemaking research projects, including yeast strain trials, yeast nutrition trials, color enhancement and stability trials for red wines and the winemaking evaluation of various vineyard research projects. He is also involved in the evaluation of new technologies for the winemaking laboratory, as well as lab proficiency training and testing in all of Beringer Blass’ wineries in California. Tannins are complex polymeric compounds that are responsible at least in part for many of the sensory attributes of wines, particularly red wines. Tannins are involved in the tactile sense of astringency in red wines, they play an important role in the intensity and stability of color in red and tannins are responsible in many cases for the brown color that develops with age in both red and white wines. The flavonoid compounds that polymerize to form some types of tannins are also thought to provide many of the health benefits associated with the moderate consumption of wines. The grape berries themselves contain some tannin, particularly in the seeds and skins. Certain types of tannins arise from polymerization of monomeric phenols during wine processing and aging. Wines aged in wood cooperage or wines that have had exposure to oak dust, chips or staves will also extract some types of tannins from the wood. Finally, pure or relative pure mixtures of tannins may be added to the wine during processing or aging. Tannins are polymers of simpler phenols, which can generally be classed as either flavonoid or nonflavonoid. The simplest of phenols is phenol itself, which consists of an aromatic ring and a single hydroxyl group (Figure 1). 32nd Annual New York Wine Industry Workshop 19 Figure 1. Phenol Catechin, (Figure 2), is an example of a flavonoid, in this case a flavan-3-ol. Flavonoids have a common structure that consists of an aromatic “A” ring, an oxygen containing heterocyclic “C” ring and a second Figure 2. Catechin, a flavan-3-ol aromatic “B” ring. There are several classes of flavonoids that differ primarily in the structure of the heterocyclic ring. From these and similar building blocks, tannins are polymerized. There are two primary classes of tannins found naturally in grapes and wine. The first class, the hydrolysable tannins, is based on non-flavonoid phenolics. The basic unit in a hydrolysable tannin is a polyhydroxy molecule, Figure 3. Gallic acid usually a sugar, to which are bound multiple non-flavonoid phenols, often gallic acid (Figure 3) or ellagic acid, which is a dimer of gallic acid (Figure 4). Gallotannin (Figure 5) is an example of a hydrolysable tannin. These tannins are readily hydrolyzed under acidic or basic conditions, hence their name. Nonflavonoid phenols and hydrolysable tannins are also found primarily in the skins and in the pulp. 32nd Annual New York Wine Industry Workshop 20 Figure 4. Ellagic acid Figure 5. Gallotannin (“G” is a gallic acid unit) The second primary class of tannins in grapes and wines are the condensed tannins. These tannins are polymers of primarily flavan-3-ols (catechin and epicatechin), along with the anthocyanin pigments. If an anthocyanin is incorporated into the tannin, the resulting polymer is often colored. As a covalent bond is formed between the individual units within a condensed tannin, these tannins are not readily hydrolyzed. Typically the linkage occurs between the 4 position (on the heterocyclic ring) and the 8 position on the aromatic A ring, although it is possible for the linkage to occur at the 6 position as well. 32nd Annual New York Wine Industry Workshop 21 Figure 6. An epicatechin tetramer In Figure 6, the tetramer consists of linkages just between the 4 position and the 8 position of the adjacent epicatechin units. These polymers can include both catechin and epicatechin units. The epicatechin and catechin monomers are found primarily in the seed coat in grapes, and to a lesser extent in the skins. Polymers of these two compounds (condensed tannins) are found in the seeds, skins and to a lesser extent, in the pulp. Monomeric anthocyanins and polymeric pigments are found primarily in the skins of red grapes. Polymerization of the condensed tannins continues as the juice is processed into wine and as the wine ages. As the number of units in the polymers increases, their color changes from colorless to yellow to brown. As the number of units increases, the solubility of the polymer decreases, and eventually the polymer will precipitate from solution. If the tannin incorporates an anthocyanin moiety, this precipitation will result in slowly declining color as the wine ages. As tannins are very complex compounds, common methods for determining their concentrations in grape tissues or wines are often indirect or empirical in nature. Comparison of results from different studies can be problematic. Most analyses of tannins begin with an attempt to differentiate them from their monomeric building blocks. This may be done either by a separation based on the larger size of the 32nd Annual New York Wine Industry Workshop 22 tannins or by selective precipitation or extraction. Once separated from the monomeric phenols, the tannins may be analyzed by a number of available methods for the analysis of phenolic compounds. The most widely used is probably the Folin-Ciocalteu colorimetric method. Gallic acid is used as the standard for this method and results are reported as gallic acid equivalents (GAE). It is not possible, given the complex mixture of tannins present in grape tissue or wine samples to convert GAE into an exact amount of tannin, but GAE are never-the-less useful as a tool for comparisons amongst samples or treatments. Using a low pressure chromatographic method for separation of non-polymeric and polymeric phenols, Kantz and Singleton (1991) reported that grape seeds contained the highest amount of polymeric phenols of the grape tissues they looked at, with polymeric phenols ranging from 21 to 27 mg GAE/g fresh weight. Polymeric phenols accounted for 60 to 70% of the total extractable phenolic content of the seeds. Grape skins contained much lower total and polymeric phenols and were much more variable, ranging from 0.1 to 5 mg GAE/g fresh weight of skins; polymeric phenols accounted for from 6% to 43% of the total extractable phenols in the skins. Thorngate and Singleton (1994) subsequently showed that most of the phenolic material in the seeds was actually in the outer layers of the seed, with very little found in the seed endosperm. Of the total pool of phenolic material available in the grape berry, only a part is subsequently found in the resulting wines. Incomplete extraction, adsorption with yeast lees and other solids, precipitation with grape or yeast proteins and a host of other reactions all tend to limit the total phenol and polymeric phenol levels in wine. Singleton and Draper (1964) studied the extraction of polymeric phenols from grape seeds into wine; complete extraction of the tannin in the seeds should result in a tannin content of 200 to 400 mg/L. Under typical red wine fermentation conditions about half that amount is common. That level is then further reduced by other reactions and by precipitation. They also showed that in the same length of time, more complete extraction is favored by increased temperature and increased ethanol content. References: Kantz, K. and V.L.Singleton. Isolation and determination of polymeric polyphenols using Sephadex LH20 and analysis of grape tissue extracts. Am. J. Enol. Vitic. 42:309-16 (1991). Singleton, V.L. and D.E. Draper. The transfer of polyphenolic compounds from grape seeds into wines. Am. J. Enol. Vitic. 15:34-40 (1964). Thorngate, J.H. and V.L. Singleton. Localization of procyanidins in grape seeds. Am. J. Enol. Vitic. 45: 259-262 (1994). 32nd Annual New York Wine Industry Workshop 23