Lecture 22.Phytochemistry1.Tannins

Phytochemistry1
Lecture 22 Date : 3/5/2015
Tannins Done By:Sanaa Otoom+
Walaa Wahdan
Hydrolysable tannins:
Are products of esterification of gallic acid with sugars .
It occur sometimes that two closed or near gallic acid residue to undergo further dimerization carbon dimerization of aromatic rings especially C# 2 this structure is called hexahydroxy diphenic acid (HHDP)
To conclude :
Hydrolysable tannins are either :
1- Gallotannins : Example: pantagalloylglucose , contains individual gallic acid without coupling with each other , if hydrolyzed in acidic media, high Temp ,
Oxygen will produce gallic acids and glucose.
2- Ellagitannins: Oxidative coupling “gallic acid dimerization” , if hydrolyzed in acidic media, high temp and oxygen will produce sugar “glucose” and
Hexahydroxydiphenic acid BUT HHDP in acidic media is unstable and undergo lactonization “ester linkage” Ellagic aicd (Dilactone).
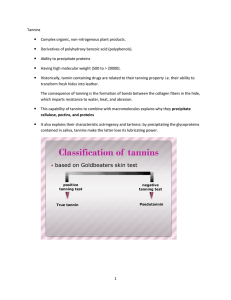
The most important plants containing Tannins are nutgall : the hymenopterous insect comes and put its egg on the oak of this plant and this process will induce the formation of large amounts of tannins. Nutgall rich in hydrolysable tannins mainly and collectively refer to as Tannic acid. (please refer to slide #20 for more details)
**Tannic acid : it’s NOT single chemical substance, it’s mixture of substances
“hydrolysable tannins” obtained via extraction method, this preparation consists of :
1- Pentagalloyl glucose
2- Pedunculagin
3- Tellimagradin
And others.
This plant also contain other substances such as free gallic or ellagic acids, sterols, triterpenes and starch but mainly consist of hydrolysable tannins.
Please refer to Extraction method in nutgall in slide #21.
Second plant is Witch hazel ةرحاسلا قدنب وا ةزوج :
1 | P a g e
Phytochemistry1
It contains tannins and proanthocyanidins.
Also contains flavonol glucoside astragalin.
Part used
the leaf
Tannins have direct vasoconstrictor effect due to their direct sympathomimetic action on vessels or from their astringent effect on capillaries.
D-Ribose
This is structure of hamamelitannins, consists of two galloyl moieties connected to sugar moiety (D-Ribose):
D-ribose at carbon number 1 contains aldehyde group , in open chain, at carbon number 2 & 5 there’s gallic acid.
One moiety of ga llic acid not esterified directly on carbon #2 , it’s esterfied on hydroxymethyl group at carbon #2.
Physiochemical properties of Tannins :
Solubility of tannins in water depends on type of tannins :
1- Hydrolysable tannins are more water soluble than condensed tannins.
2 | P a g e
Phytochemistry1
2- Solubility of condensed tannins also depends on degree of polymerization : dimeric are more soluble than polymeric.
Extraction: we depend on principle like dissolves like.
From the Dr “you have to search about, expected question”, Important Question: why we don’t use methanol in the extraction of tannins?
Answer (Not 100% sure): it depends on polarity of methanol and the compounds are extracted.
**the most important step toward separation is extraction.
Characterization :
Using color reaction; by developing color with the same reagent used after TLC separation for visualization purposes.
In pharmacopeia the monograph contain two important sections; one for the raw material and the other for the product (drug product, herbal or non-herbal): identification (qualitative) section and assay (quantitative) section.
>>>> In the identification we have to confirm the presence of drug (active constituent), the most utilizable tool in identification is IR (by comparing the IR spectrum obtain for the sample with the spectrum of chemical reference standard.
** Always we must have a reference standard whether in quantification or qualitative.
Always IR supported by TLC especially for mixtures (product and yield).
Nowadays we can measure the IR for the sample even in the mixture, in drug formulation (powder, cream).
TLC separate the mixture (we make applications one for the sample and the other for standard, then put it in the mobile phase >>> will separate, we compare the spots obtain from sample with the one obtained from the standard (the spots must be matched) in mean of distance traveled and color developed.
Always after separation with TLC, you have to dry the spots and spray with chemical reagent (locating, localizing, visualizing or identification reagent) in order to visualize the spot.
>>>> So in visualizing either the substance is colored like tannins, anthocyanins or in most cases you need to spray it with chemical reagent to obtain a color reaction. You also could see it under UV radiation if the substance is florescent one (will excited by UV
3 | P a g e
Phytochemistry1 radiation to give color), sometimes it need chemical treatment before visualizing under UV.
** ellagitannins + nitrous acid (+acetic acid) >>> will give range of colors, depending on the nature of ellagitannins and its concentration.
** RP-HPLC >>> reversed phase HPLS.
Quantitation:
Assay part to confirm how much of the active ingredient we have in comparison with the claimed amount.
Also the qualitative technique could be utilized for quantitation, when measure the color by UV (absorption)>>> calorimetric determination.
Many methods used:
1- Protein precipitation technique (most common used): 2 method according to the protein used:
A) Hemoglobin precipitation (blood method):
The principle depend on the precipitation of Hb that composed of heme + globin (protein) by tannins (sample), we used extra
(excess) Hb standard preparation (not all will precipitate), also we make dilution for tannins preparation.
Excess Hb >>>> part will precipitate in the tannin and the rest remain free (unprecipitated).
Total – uncombined (measured by calorimetric technique)
=combined (tannins) >>> for sample
Do the same with the standard >>> will give the combined Hb with known conc
) ةنيعلل زيكرتلا دجون يلدابتلا برضلا مادختساب( a) Hide powder method:
Using proteins of animal hides.
** Total soluble matter = precipitate (resemble all the substance that was dissolved in the water, tannins + other substances).
** The two samples with same volumes.
4 | P a g e
-
Phytochemistry1
The difference between two residues resemble the weight of tannins
(tannin with the precipitated preotein), by comparing it with the output of standard (with known concentration).
Ex: tannin standard conc. = 2% Mcg …… difference in weight= 0.5g
Sample difference in weight= 1g
Then sample concentration will equal??
4%Mcg )يلدابت برض( :P
Good luck
5 | P a g e